Archive for the ‘Historical’ Category
Sunday, June 5th, 2011
In 1923, Coster and von Hevesy[1] claimed discovery of the element Hafnium, atomic number 72 (latin Hafnia, meaning Copenhagen, where the authors worked) on the basis of six lines in its X-ray spectrum. The debate had long raged as to whether (undiscovered) element 72 belonged to the rare-earth group 3 of the periodic table below yttrium, or whether it should be placed in group 4 below zirconium. Establishing its chemical properties finally placed it in group 4. Why is this apparently arcane and obscure re-assignment historically significant? Because, in June 1922, in Göttingen, Niels Bohr had given a famous series of lectures now known as the Bohr Festspiele on the topic of his electron shell theory of the atom. Prior to giving these lectures he had submitted his collected thoughts in January 1922[2].
(more…)
References
-
D. COSTER, and G. HEVESY, "On the Missing Element of Atomic Number 72", Nature, vol. 111, pp. 79-79, 1923. http://dx.doi.org/10.1038/111079a0
-
N. Bohr, "Der Bau der Atome und die physikalischen und chemischen Eigenschaften der Elemente", Zeitschrift f�r Physik, vol. 9, pp. 1-67, 1922. http://dx.doi.org/10.1007/BF01326955
Tags:Bohr, Bury, chemical bonding, Chemical IT, chemical properties, Copenhagen, General, green technologies, Hafnium, Historical, Langmuir, Niels Bohr, silicon chips, Technetium, X-ray
Posted in Uncategorised | 5 Comments »
Monday, May 9th, 2011
Introductory organic chemistry invariably features the mechanism of haloalkane solvolysis, and introduces both the Sn1 two-step mechanism, and the Sn2 one step mechanism to students. They are taught to balance electronic effects (the stabilization of carbocations) against steric effects in order to predict which mechanism prevails. It was whilst preparing a tutorial on this topic that I came across what was described as the special case of neopentyl bromide, the bimolecular solvolysis of which has been identified (DOI: 10.1021/ja01182a117) as being as much as 3 million times slower than methyl bromide. This is attributed to a very strong steric effect on the reaction, greater even than that which might be experienced by t-butyl bromide! Time I thought, to take a look at what might make neopentyl bromide so special, and what those supposed electronic and steric effects were really up to.
(more…)
Tags:free energy, free energy barrier, Historical, potential energy surface, Tutorial material
Posted in Uncategorised | 1 Comment »
Sunday, April 17th, 2011
The structure of ferrocene was famously analysed by Woodward and Wilkinson in 1952[1],[2], symmetrically straddled in history by Pauling (1951) and Watson and Crick (1953). Quite a trio of Nobel-prize winning molecular structural analyses, all based on a large dose of intuition. The structures of both proteins and DNA succumbed to models built from simple Lewis-type molecules with covalent (and hydrogen) bonds; ferrocene is intriguingly similar and yet different. Similar because e.g. carbon via four electron pair bonds. He did not (in 1916) realise that 8 = 2(1 + 3), and that the next in sequence would be 18 = 2(1 + 3 + 5). That would have to wait for quantum mechanics, and of course inorganic chemists now call it the 18-electron rule (for an example of the 32-electron rule, or 2+6+10+14, as first suggested by Langmuir in 1921[3] (see also here[4]).
(more…)
References
-
G. Wilkinson, M. Rosenblum, M.C. Whiting, and R.B. Woodward, "THE STRUCTURE OF IRON BIS-CYCLOPENTADIENYL", Journal of the American Chemical Society, vol. 74, pp. 2125-2126, 1952. http://dx.doi.org/10.1021/ja01128a527
-
G. Wilkinson, "The iron sandwich. A recollection of the first four months", Journal of Organometallic Chemistry, vol. 100, pp. 273-278, 1975. http://dx.doi.org/10.1016/S0022-328X(00)88947-0
-
I. Langmuir, "Types of Valence", Science, vol. 54, pp. 59-67, 1921. http://dx.doi.org/10.1126/science.54.1386.59
-
J. Dognon, C. Clavaguéra, and P. Pyykkö, "Towards a 32‐Electron Principle: Pu@Pb12 and Related Systems", Angewandte Chemie International Edition, vol. 46, pp. 1427-1430, 2007. http://dx.doi.org/10.1002/anie.200604198
Tags:10.1126, 18-electron rule, 54.1386.59, Ferrocene, Historical, Interesting chemistry, normal solution, pence, Tutorial material, valid search query
Posted in Uncategorised | 8 Comments »
Saturday, April 9th, 2011
Understanding why and how proteins fold continues to be a grand challenge in science. I have described how Wrinch in 1936 made a bold proposal for the mechanism, which however flew in the face of much of then known chemistry. Linus Pauling took most of the credit (and a Nobel prize) when in a famous paper[1] in 1951 he suggested a mechanism that involved (inter alia) the formation of what he termed α-helices. Jack Dunitz in 2001[2] wrote a must-read article[3] on the topic of “Pauling’s Left-handed α-helix” (it is now known to be right handed). I thought I would revisit this famous example with a calculation of my own and here I have used the ωB97XD/6-311G(d,p) DFT procedure[4] to calculate some of the energy components of a small helix comprising (ala)6 in both left and right handed form.
(more…)
References
-
L. Pauling, R.B. Corey, and H.R. Branson, "The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain", Proceedings of the National Academy of Sciences, vol. 37, pp. 205-211, 1951. http://dx.doi.org/10.1073/pnas.37.4.205
-
J.D. Dunitz, "Pauling's Left-Handed α-Helix", Angewandte Chemie International Edition, vol. 40, pp. 4167-4173, 2001. http://dx.doi.org/10.1002/1521-3773(20011119)40:22<4167::AID-ANIE4167>3.0.CO;2-Q
-
K.S. Thanthiriwatte, E.G. Hohenstein, L.A. Burns, and C.D. Sherrill, "Assessment of the Performance of DFT and DFT-D Methods for Describing Distance Dependence of Hydrogen-Bonded Interactions", Journal of Chemical Theory and Computation, vol. 7, pp. 88-96, 2010. http://dx.doi.org/10.1021/ct100469b
Tags:alpha-helix, aqueous solutions, chiroptical, conformational analysis, dielectric, energy, energy components, high energy species, Historical, Interesting chemistry, Jack Dunitz, Julia Contreras-Garcia, protein, solvation algorithms, Tutorial material, watoc11
Posted in Uncategorised | 4 Comments »
Monday, April 4th, 2011
Andy Mclean posted a comment to my story of copper phthalocyanine (Monastral blue). The issue was its colour, and more specifically why this pigment has two peaks λmax 610 and 710nm making it blue. The first was accurately reproduced by calculation on the monomer, but the second was absent with such a model. Andy suggested this latter was due to stacking. Here, the calculated spectrum of a stacked dimer is explored.
(more…)
Tags:Andy Mclean, copper phthalocyanine, energy, Historical, Interesting chemistry, TD-DFT, X-ray
Posted in Uncategorised | No Comments »
Monday, April 4th, 2011
Most scientific theories emerge slowly, over decades, but others emerge fully formed virtually overnight as it were (think Einstein in 1905). A third category is the supernova type, burning brightly for a short while, but then vanishing (almost) without trace shortly thereafter. The structure of DNA (of which I have blogged elsewhere) belongs to the second class, whilst one of the brightest (and now entirely forgotten) examples of the supernova type concerns the structure of proteins. In 1936, it must have seemed a sure bet that the first person to come up with a successful theory of the origins of the (non-random) relatively rigid structure of proteins would inevitably win a Nobel prize. Of course this did happen for that other biologically important system, DNA, some 17 years later. Compelling structures for larger molecules providing reliable atom-atom distances based on crystallography were still in the future in 1936, and so structural theories contained a fair element of speculation and hopefully inspired guesswork (much as cosmological theories appear to have nowadays!).
(more…)
Tags:Cambridge, chair, Derek Barton, Dorothy Wrinch, energy, high energy species, Historical, Interesting chemistry, mathematician, organic chemist, Patrick Coffey, relative free energy, thermodynamics, Tutorial material
Posted in Uncategorised | 2 Comments »
Tuesday, March 8th, 2011
The story of Monastral is not about a character in the Magic flute, but is a classic of chemical serendipity, collaboration between industry and university, theoretical influence, and of much else. Fortunately, much of that story is actually recorded on film (itself a unique archive dating from 1933 and being one of the very first colour films in existence!). Patrick Linstead, a young chemist then (he eventually rose to become rector of Imperial College) tells the story himself here. It is well worth watching, if only for its innocent social commentary on the English class system (and an attitude to laboratory safety that should not be copied nowadays). Here I will comment only on its colour and its aromaticity.
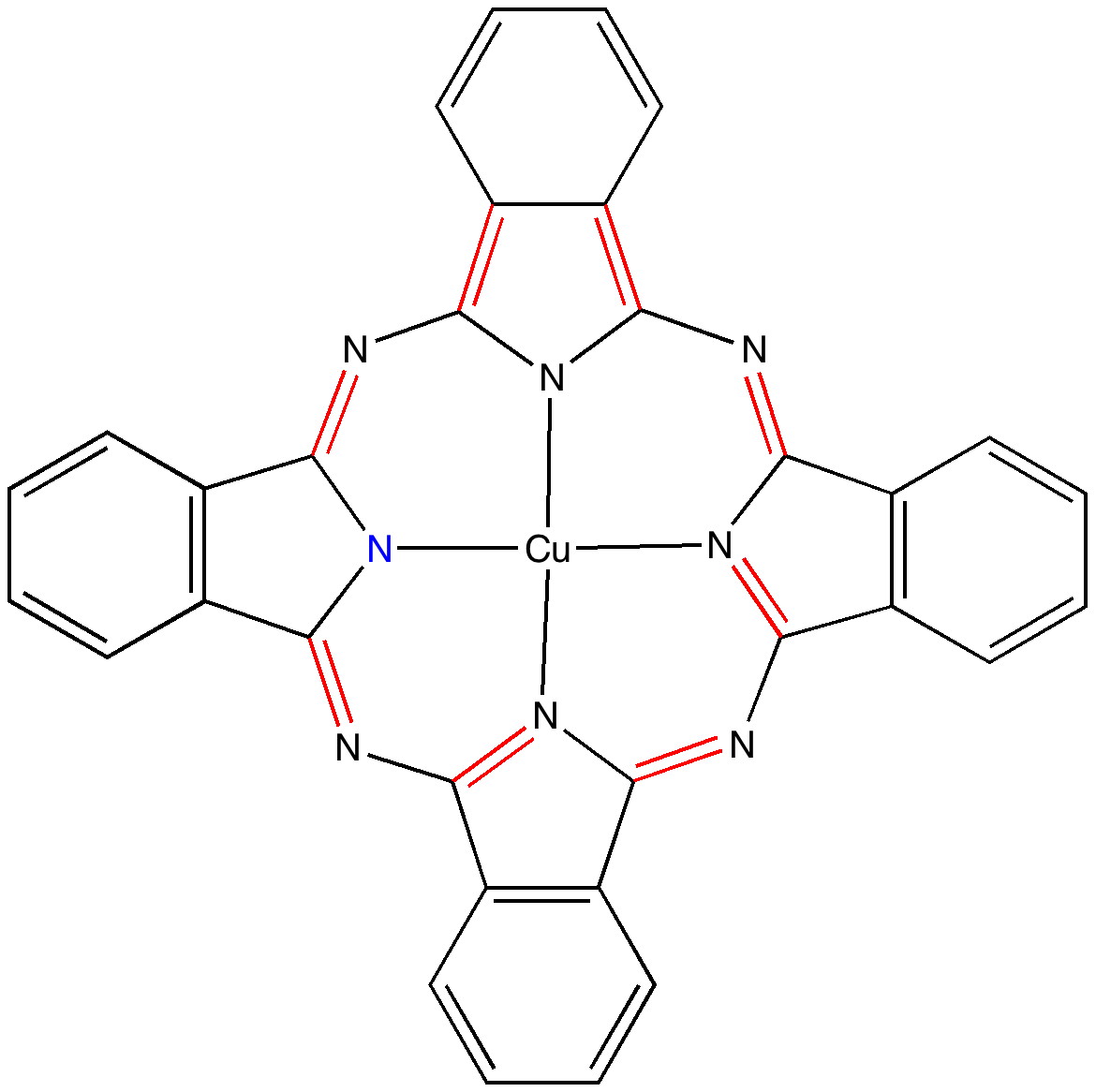
Copper phthalocyanine
(more…)
Tags:18 electron aromaticity, chemical serendipity, Historical, HTML, HTML element, Imperial College, Interesting chemistry, Missouri, Monastral blue, Patrick Linstead, phthalocyanine, Phthalocyanine Blue BN, Phthalocyanines, Pigments, rector, young chemist
Posted in Uncategorised | 6 Comments »
Thursday, February 24th, 2011
One of my chemical heroes is William Perkin, who in 1856 famously (and accidentally) made the dye mauveine as an 18 year old whilst a student of August von Hofmann, the founder of the Royal College of Chemistry (at what is now Imperial College London). Perkin went on to found the British synthetic dyestuffs and perfumeries industries. The photo below shows Charles Rees, who was for many years the Hofmann professor of organic chemistry at the very same institute as Perkin and Hofmann himself, wearing his mauveine tie. A colleague, who is about to give a talk on mauveine, asked if I knew why it was, well so very mauve. It is a tad bright for today’s tastes!
(more…)
Tags:August von Hofmann, Charles Rees, chemical heroes, chiroptical, colour, founder, General, Historical, Hofmann, HOMO, Imperial College, Imperial College London, Interesting chemistry, LUMO, Mauveine, Perkin, professor of organic chemistry, purple, Rees, Royal College of Chemistry, William Perkin
Posted in Uncategorised | 16 Comments »
Saturday, February 5th, 2011
In 1953, the model of the DNA molecule led to what has become regarded as the most famous scientific diagram of the 20th century. It had all started 93 years earlier in 1860, at a time when the tetravalency of carbon was only just established (by William Odling) and the concept of atoms as real entities was to remain controversial for another 45 years (for example Faraday, perhaps the most famous scientist alive in 1860 did not believe atoms were real). So the idea of constructing a molecular model from atoms as the basis for understanding chemical behaviour was perhaps bolder than we might think. It is shown below, part of a set built for August Wilhelm von Hofmann as part of the lectures he delivered at the Royal College of Chemistry in London (now Imperial College).
(more…)
Tags:and compare the two, chemical behaviour, deficiency, General, Hermann Sachse, Historical, Hofmann, Imperial College, Josef Loschmidt, Loschmidt, model, modern model, molecular model, Odling, Royal College of Chemistry, Royal College of Chemistry in London, Sachse, scientist, Tutorial material, Wilhelm von Hofmann, William Odling
Posted in Uncategorised | 3 Comments »
Wednesday, December 29th, 2010
Science is about making connections. Plenty are on show in Watson and Crick’s famous 1953 article on the structure of DNA[1] but often with the tersest of explanations. Take for example their statement “Both chains follow right-handed helices“. Where did that come from? This post will explore the subtle implications of that remark (and how in one aspect they did not quite get it right!).
(more…)
References
-
J.D. WATSON, and F.H.C. CRICK, "Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid", Nature, vol. 171, pp. 737-738, 1953. http://dx.doi.org/10.1038/171737a0
Tags:Bijvoet, chemist, chiroptical, d(CGCG), Derek Barton, dispersion forces, DNA duplex, Historical, Interesting chemistry, Marcus du Sautoy, Note, Odile Crick, professional artist, Tartaric Acid, van der Waals, watoc11, Watson Crick, Web sense, Z-DNA
Posted in Uncategorised | 8 Comments »
