What constitutes an “impossible molecule”? Well, here are two, the first being the topic of a recent article[1]. The second is a favourite of organic chemistry tutors, to see if their students recognise it as an unusual (= impossible) form of a much better known molecule.
Perhaps we could define impossible molecules into two slightly different classes.
- The first class is a molecule which is entirely normal in terms of its structure and bonding, but just happens to be thermodynamically less stable than an isomeric form. If all mechanistic possibilities for converting it to the more stable form are eliminated, then there is no reason it should not be detected, even though it is “impossible”. By the way, quite a number of impossible molecules have been prepared using sterics (t-butyl groups and the like, a strategy first used perhaps 40 or so years ago) to prevent the molecule from either reacting with itself or with other molecules.
- The second class is a molecule where the bonding or its structure are so deviant from accepted theories of the structures of molecules that its energy is either so high that either it simply cannot be prepared in the first place, or that nothing can be done to prevent its rearrangement to a much more stable form.
The first of the examples below falls clearly into the first category; methane triol. As reported[1], this impossible molecule has now been detected both at low temperatures and in the gas phase at low pressure using time-of-flight mass spectrometry and other elegant experiments. The key is to ensure either a very low temperature or the absence of any acid catalyst to decompose it to methanol and formic acid.
As is my usual practice in discussing any interesting molecule, I first tend to conduct a search of the CSD (Cambridge structure database) – in this case it has to be said with little hope of finding any examples. I was therefore very surprised to find one example reported, COLRUT.[2] The crystal structure of COLRUT can be viewed here.[3] (DOI: 10.5517/ccdc.csd.cc22yztvv). Clearly, given the discussion at the top, alarm bells should be ringing about this result. When any such alarms sound, it is my second practice to turn to calculations for verification. In this case to FAIR Data calculations[4] (DOI: 10.14469/hpc/14236).
The article[1] also reports such calculations, but its good to have independent verification (of some of them), so I list the essential conclusions from my own calculations here.
- At the CCSD(T)/Def2-TZVPP level, methane triol is ΔG298 14.49 kcal/mol higher in free energy than formic acid and water. This is not really an impossibly higher energy, and the molecule is “impossible” only because there is a very facile reaction for it to undergo (acid catalysed disproportionation for example).
- At the much faster ωB97X-D/Def2-TZVPP level, the value is 14.48 kcal/mol, which is agrees well enough with the previous to use this method to explore further.
- If the C-H is replaced by C-CF3 (again a good tutorial question for how to stabilize the diol form of eg acetone), the energy of the triol is reduced to +9.4 kcal/mol. Still positive, but much smaller than the original.
- If the C-H is replaced by C-(CF3)3 it is still unstable by 13.6 kcal/mol. Not much chance of using substituents to create a “possible” triol then.
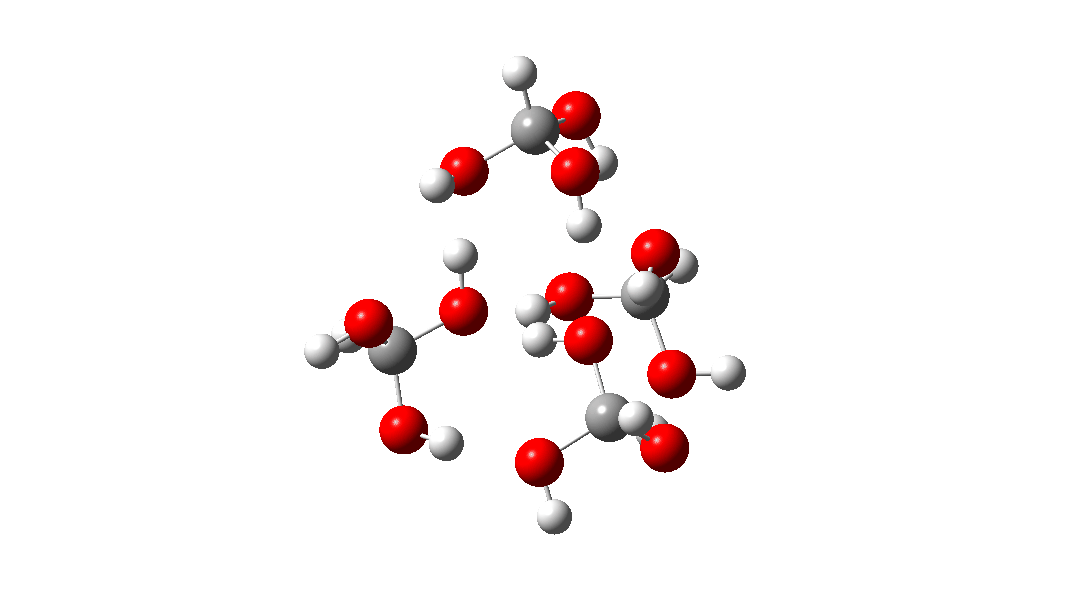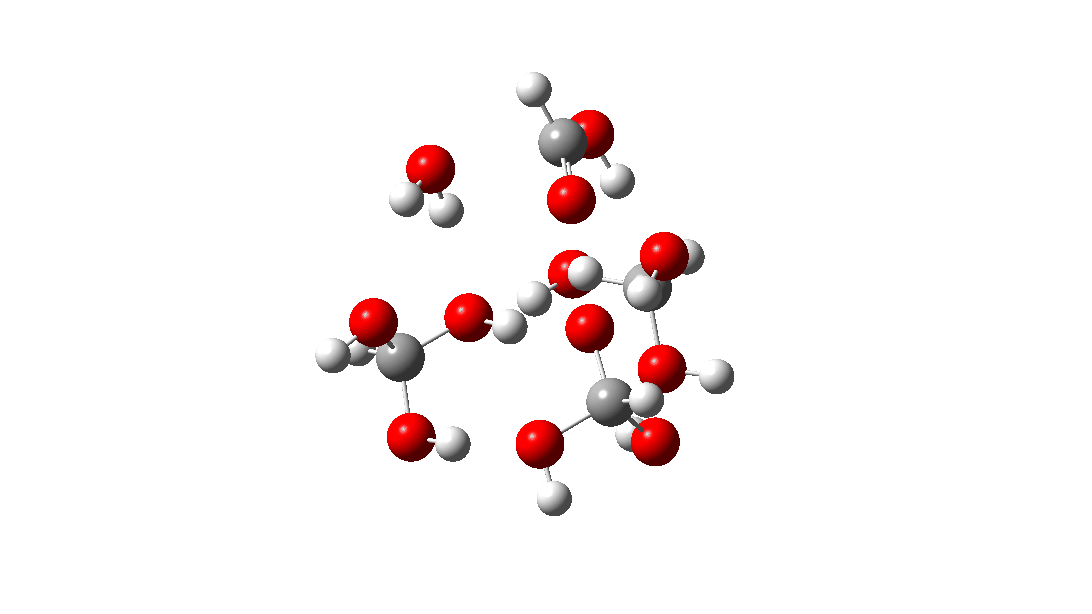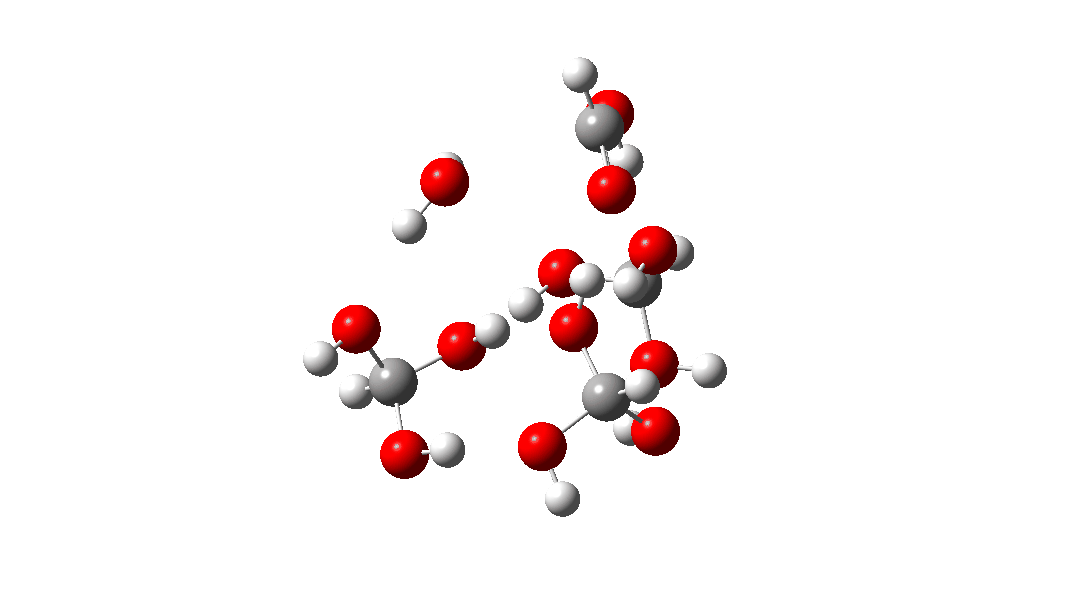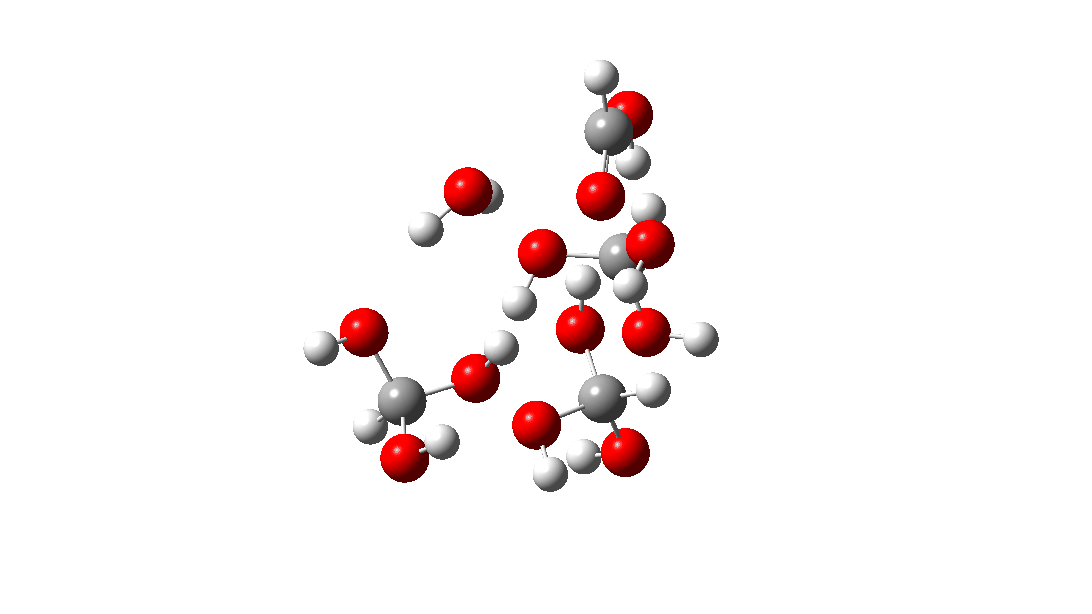
- Next, the transition state for unimolecular decomposition to water and formic acid. An IRC for this is shown below and the free energy of activation is +36.6 kcal/mol. This proceeds via a very non-linear hydrogen transfer, a geometry known to be unfavourable and indeed an energy too high for this rearrangement to occur (in a mass spectrometer? What is the temperature of molecules under these conditions?). Note how a nice hydrogen-bonded form of the products forms at the end.
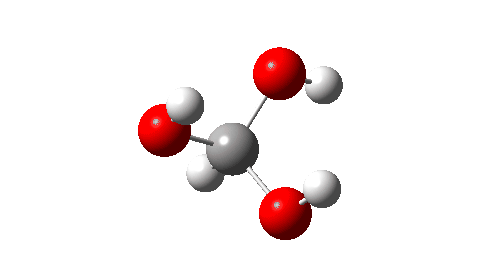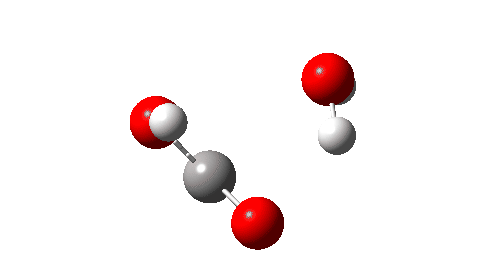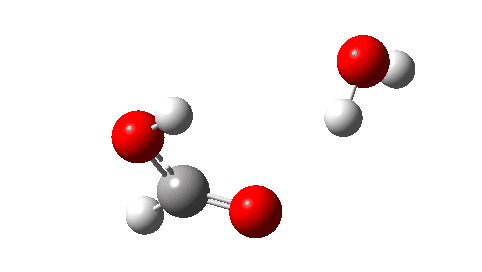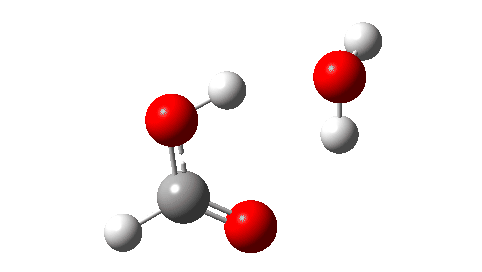
I could not resist showing the dipole moment response along the IRC. Lovely! - What about an intermolecular rearrangement, which would occur at either higher pressures or perhaps higher temperatures? Now, ΔG‡ = 26.7 kcal/mol, a more viable thermal reaction.The lower barrier is because the 6-ring transition state now allows a less bent hydrogen transfer.
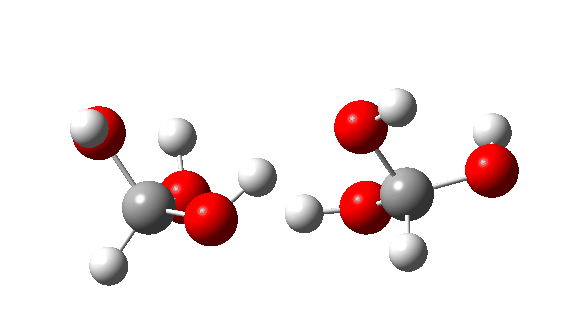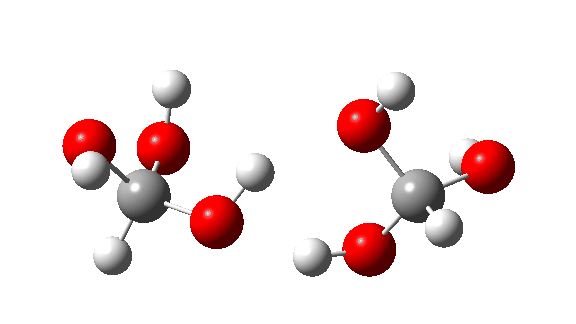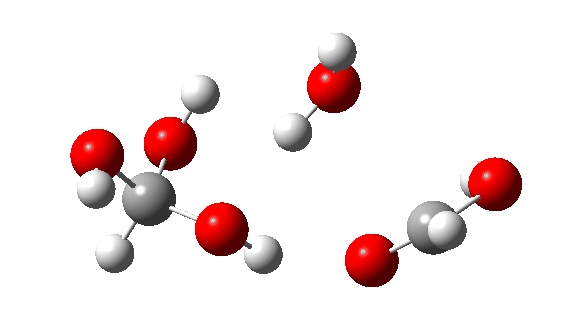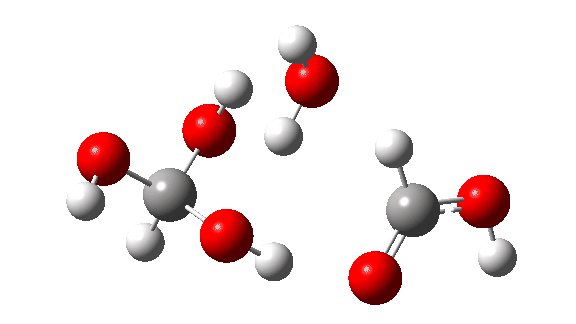
- This is the reaction of a trimer, ΔG‡ = 24.2 kcal/mol. The 8-ring transition state now allows almost linear hydrogen transfers. Note that all three transferring hydrogens move more or less in synchrony.
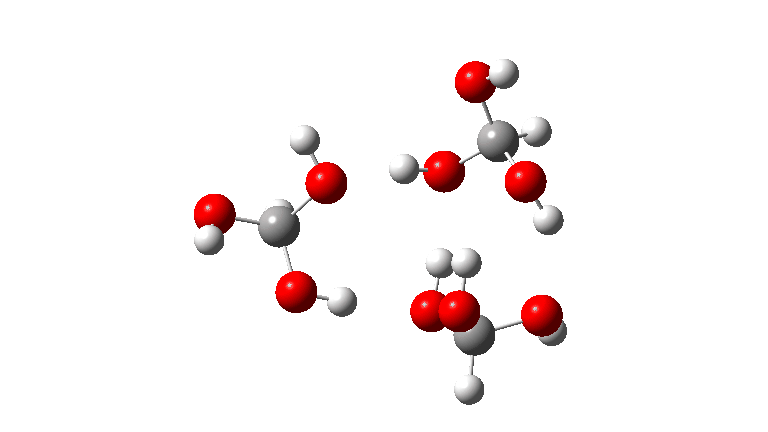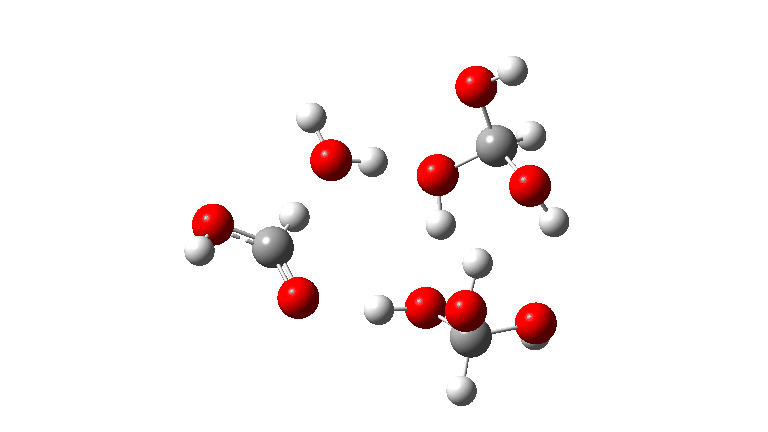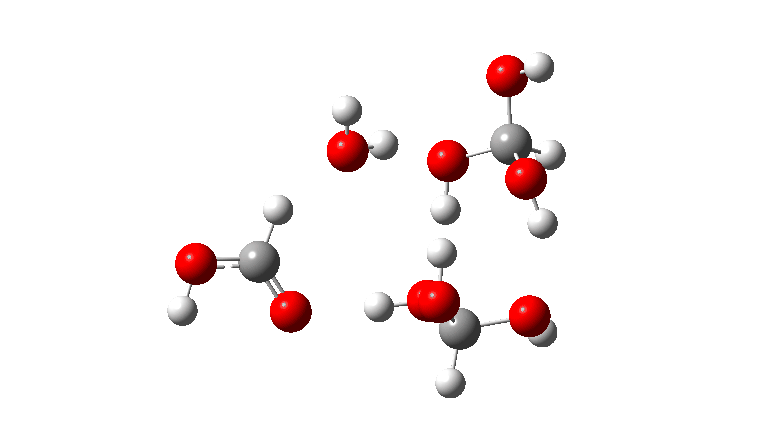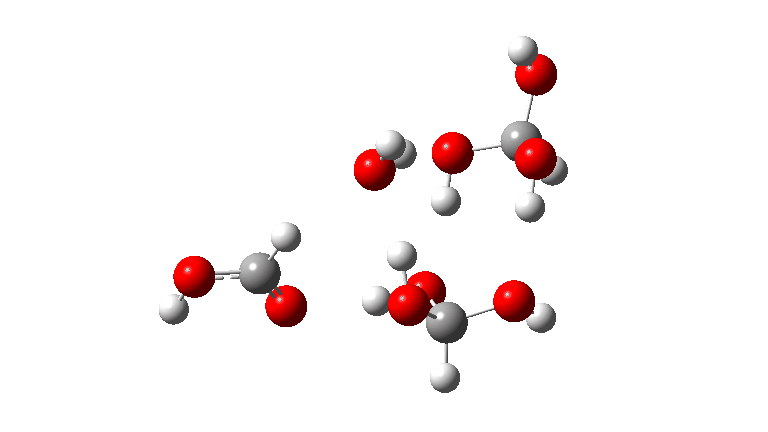
- The tetramer: ΔG‡ = 24.1 kcal/mol, now via a 10-ring transition state. If you look carefully at the animation, you can now see that the hydrogen transfers have become very non-synchronous (and the transition state more ionic), although they remain almost linear.
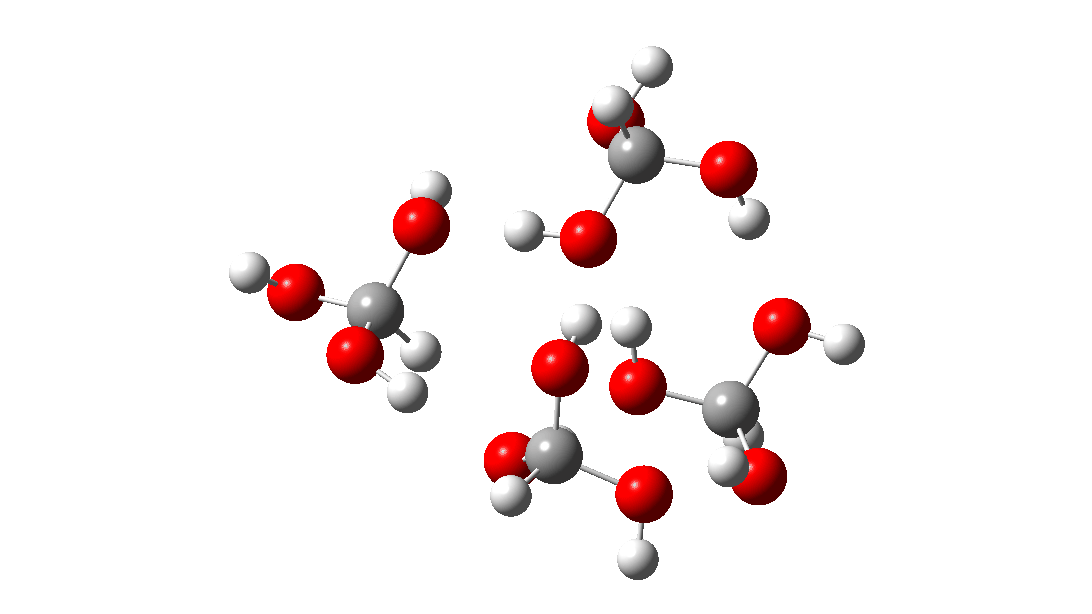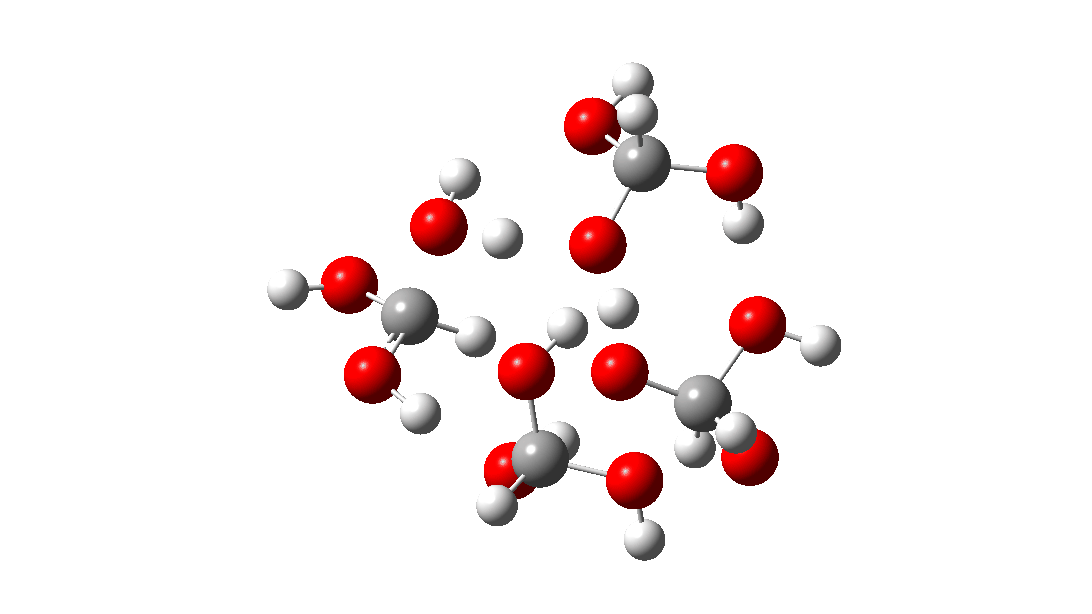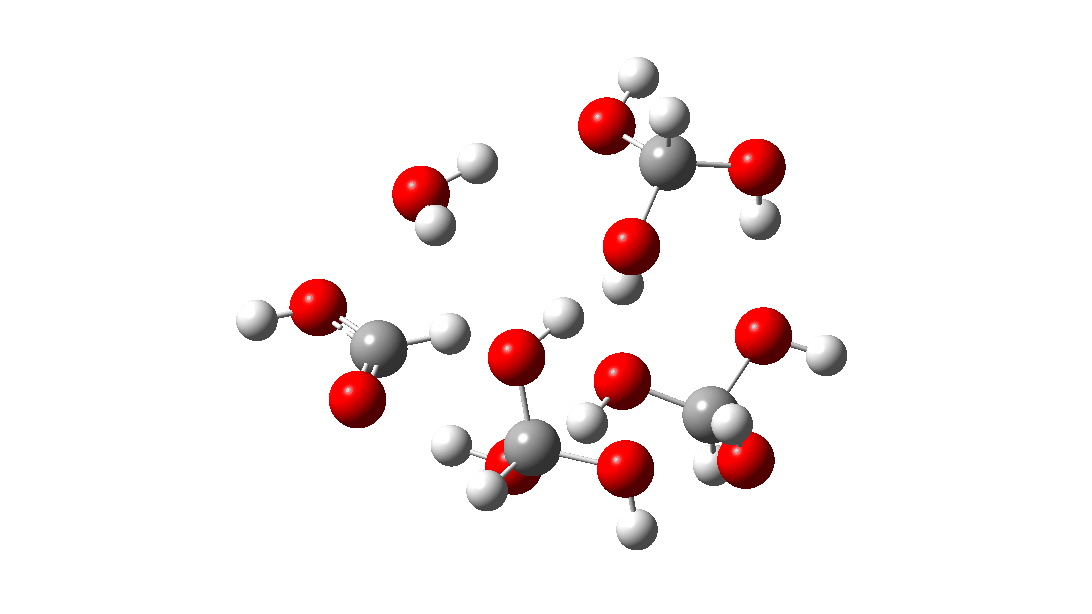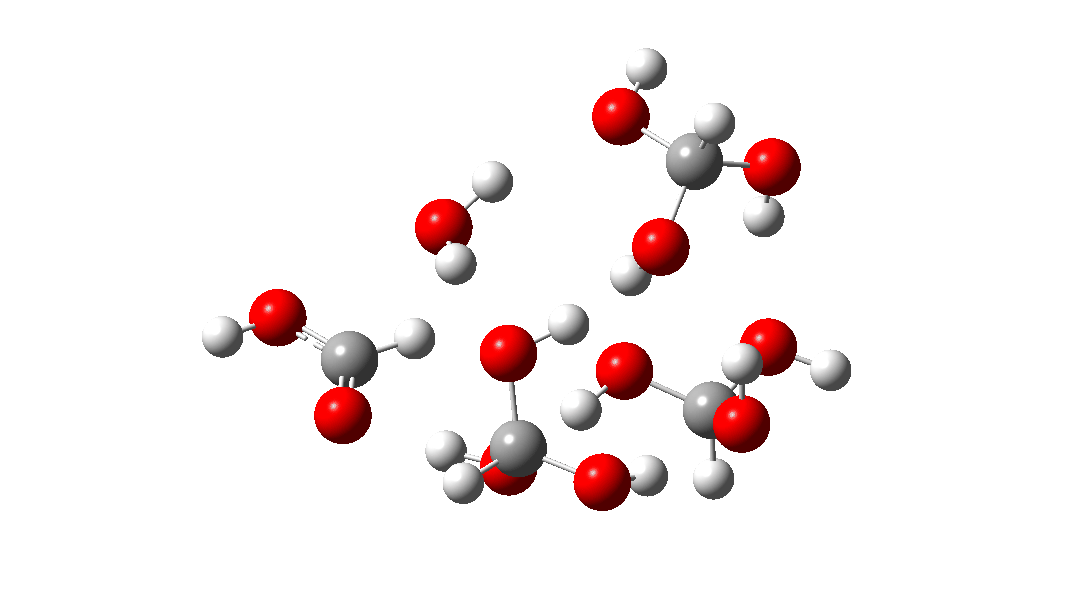
- But wait, there is another isomer of the tetramer reaction, instead proceeding via an 8-ring TS, with the fourth triol molecule bonding to the transition state via four hydrogen bonds. This is very much like a stabilised protein transition state and overcomes the extra entropy of adding that fourth molecule and then some; ΔG‡ = 18.9 kcal/mol. So at high concentrations the disproportionation of methane triol is predicted to become a facile reaction and now can only be prevented at low temperatures!
<
An NCI (non-covalent-interaction) analysis of the hydrogen bonds in this TS structure is shown below. The blue regions are hydrogen bonds. The ones labelled 1-4 are the four such interactions resulting from addition of a fourth molecule to the hydrogen transfer structure of the trimer. Click for a 3D rotatable model.

So I hope this extended analysis of what makes an “impossible molecule” actually possible adds another dimension to the original report.[1] As for that crystal structure, I will report to CCDC that it may in fact be an artefact and that they should take another look at the crystal structure data and correct it if needed. It is also interesting to explore the properties of cyclic hydrogen transfer reactions. The conclusion here is that an 8-ring transfer may be optimum, especially if it can be stabilized with four or more hydrogen bonds!
References
- J.H. Marks, X. Bai, A.A. Nikolayev, Q. Gong, C. Zhu, N.F. Kleimeier, A.M. Turner, S.K. Singh, J. Wang, J. Yang, Y. Pan, T. Yang, A.M. Mebel, and R.I. Kaiser, "Methanetriol─Formation of an Impossible Molecule", Journal of the American Chemical Society, vol. 146, pp. 12174-12184, 2024. https://doi.org/10.1021/jacs.4c02637
- P. Mi, L. He, T. Shen, J.Z. Sun, and H. Zhao, "A Novel Fluorescent Skeleton from Disubstituted Thiochromenones via Nickel-Catalyzed Cycloaddition of Sulfobenzoic Anhydrides with Alkynes", Organic Letters, vol. 21, pp. 6280-6284, 2019. https://doi.org/10.1021/acs.orglett.9b02161
- https://doi.org/
- H. Rzepa, "Exploring Methanetriol - "the Formation of an Impossible Molecule"", 2024. https://doi.org/10.14469/hpc/14236
The HC(OH)3 in the X-Ray of COLRUT is likely a chloroform molecule… or a CDCl3, as often happens for reasons obvious to chemists working in the lab.
The measured bond length “C–OH” is 180 pm, rather than the 145 pm expected; C–Cl should be in the order of 176 pm, which fits the measured value.
The C–H bond of the alleged methanetriol forms a hydrogen bond with the carbonyl oxygen, which makes sense for chloroform. Conversely the three apparent –OH are not involved in any hydrogen bonds, which would fit for –Cl instead.
Presumably the result of a somewhat lazy crystallography.
Indeed so Lukas. I reported this structure to CCDC a few weeks back, and no doubt they will make an announcement regarding it at some stage.