Linear free energy relationships (LFER) are associated with the dawn of physical organic chemistry in the late 1930s and its objectives in understanding chemical reactivity as measured by reaction rates and equilibria.
Posts Tagged ‘chemist’
Free energy relationships and their linearity: a test example.
Sunday, January 13th, 2019Early “curly” (reaction) arrows. Those of Ingold in 1926.
Wednesday, August 22nd, 2018In 2012, I wrote a story of the first ever reaction curly arrows, attributed to Robert Robinson in 1924. At the time there was a great rivalry between him and another UK chemist, Christopher Ingold, with the latter also asserting his claim for their use. As part of the move to White City a lot of bookshelves were cleared out from the old buildings in South Kensington, with the result that yesterday a colleague brought me a slim volume they had found entitled The Journal of the Imperial College Chemical Society (Volume 6).‡
Is (hν)3 an allotrope of light?
Friday, February 23rd, 2018A little while ago I pondered allotropic bromine, or Br(Br)3. But this is a far wackier report[1] of a molecule of light.
References
- Q. Liang, A.V. Venkatramani, S.H. Cantu, T.L. Nicholson, M.J. Gullans, A.V. Gorshkov, J.D. Thompson, C. Chin, M.D. Lukin, and V. Vuletić, "Observation of three-photon bound states in a quantum nonlinear medium", Science, vol. 359, pp. 783-786, 2018. https://doi.org/10.1126/science.aao7293
PIDapalooza 2018: the open festival for persistent identifiers.
Tuesday, November 14th, 2017PIDapalooza is a new forum concerned with discussing all things persistent, hence PID. You might wonder what possible interest a chemist might have in such an apparently arcane subject, but think of it in terms of how to find the proverbial needle in a haystack in a time when needles might look all very similar. Even needles need descriptions, they are not all alike and PIDs are a way of providing high quality information (metadata) about a digital object.
The demographics of a blog readership – updated
Thursday, January 8th, 2015About two years ago, I posted on the distribution of readership of this blog. The passage of time has increased this from 144 to 176 countries. There are apparently between 189-196 such, so not quite yet complete coverage! 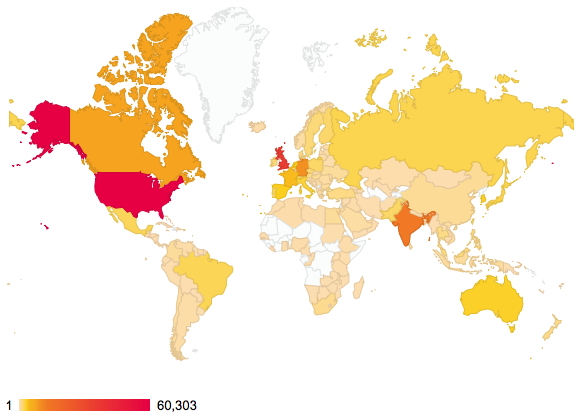
Of course, it is the nature of the beast that whilst we can track countries, very little else is known about such readerships. Is the readership young or old, student or professor, chemist or not (although I fancy the latter is less likely). Another way of keeping tabs on some of the activity are aggregators such as Chemical Blogspace, which has been rather quiet recently. Perhaps we have become too obsessed by metrics, and with the Internet-of-things apparently the “next-big-thing”, the metrics are only likely to increase. This will only encourage “game playing“, and I urge you to see a prime example of this in the UK REF (research excellence framework), the measure which attempts to rank UK universities in terms of their “excellence”.
Chemistry in the early 1960s: a reminiscence.
Monday, December 22nd, 2014I started chemistry with a boxed set in 1962. In those days they contained serious amounts of chemicals, but I very soon ran out of most of them. Two discoveries turned what might have been a typical discarded christmas present into a lifelong career and hobby.
Ribulose-1,5-bisphosphate + carbon dioxide → carbon fixation!
Sunday, April 20th, 2014Ribulose-1,5-bisphosphate reacts with carbon dioxide to produce 3-keto-2-carboxyarabinitol 1,5-bisphosphate as the first step in the biochemical process of carbon fixation. It needs an enzyme to do this (Ribulose-1,5-bisphosphate carboxylase/oxygenase, or RuBisCO) and lots of ATP (adenosine triphosphate, produced by photosynthesis). Here I ask what the nature of the uncatalysed transition state is, and hence the task that might be facing the catalyst in reducing the activation barrier to that of a facile thermal reaction. I present my process in the order it was done‡.
More joining up of pieces. Stereocontrol in the ring opening of cyclopropenes.
Thursday, July 12th, 2012Years ago, I was travelling from Cambridge to London on a train. I found myself sitting next to a chemist, and (as chemists do), he scribbled the following on a piece of paper. When I got to work the next day Vera (my student) was unleashed on the problem, and our thoughts were published[1]. That was then.
References
- M.S. Baird, J.R. Al Dulayymi, H.S. Rzepa, and V. Thoss, "An unusual example of stereoelectronic control in the ring opening of 3,3-disubstituted 1,2-dichlorocyclopropenes", Journal of the Chemical Society, Chemical Communications, pp. 1323, 1992. https://doi.org/10.1039/c39920001323
Shared space (in science).
Friday, January 6th, 2012I thought I would launch the 2012 edition of this blog by writing about shared space. If you have not come across it before, it is (to quote Wikipedia), “an urban design concept aimed at integrated use of public spaces.” The BBC here in the UK ran a feature on it recently, and prominent in examples of shared space in the UK was Exhibition Road. I note this here on the blog since it is about 100m from my office.