The story of Monastral is not about a character in the Magic flute, but is a classic of chemical serendipity, collaboration between industry and university, theoretical influence, and of much else. Fortunately, much of that story is actually recorded on film (itself a unique archive dating from 1933 and being one of the very first colour films in existence!). Patrick Linstead, a young chemist then (he eventually rose to become rector of Imperial College) tells the story himself here. It is well worth watching, if only for its innocent social commentary on the English class system (and an attitude to laboratory safety that should not be copied nowadays). Here I will comment only on its colour and its aromaticity.
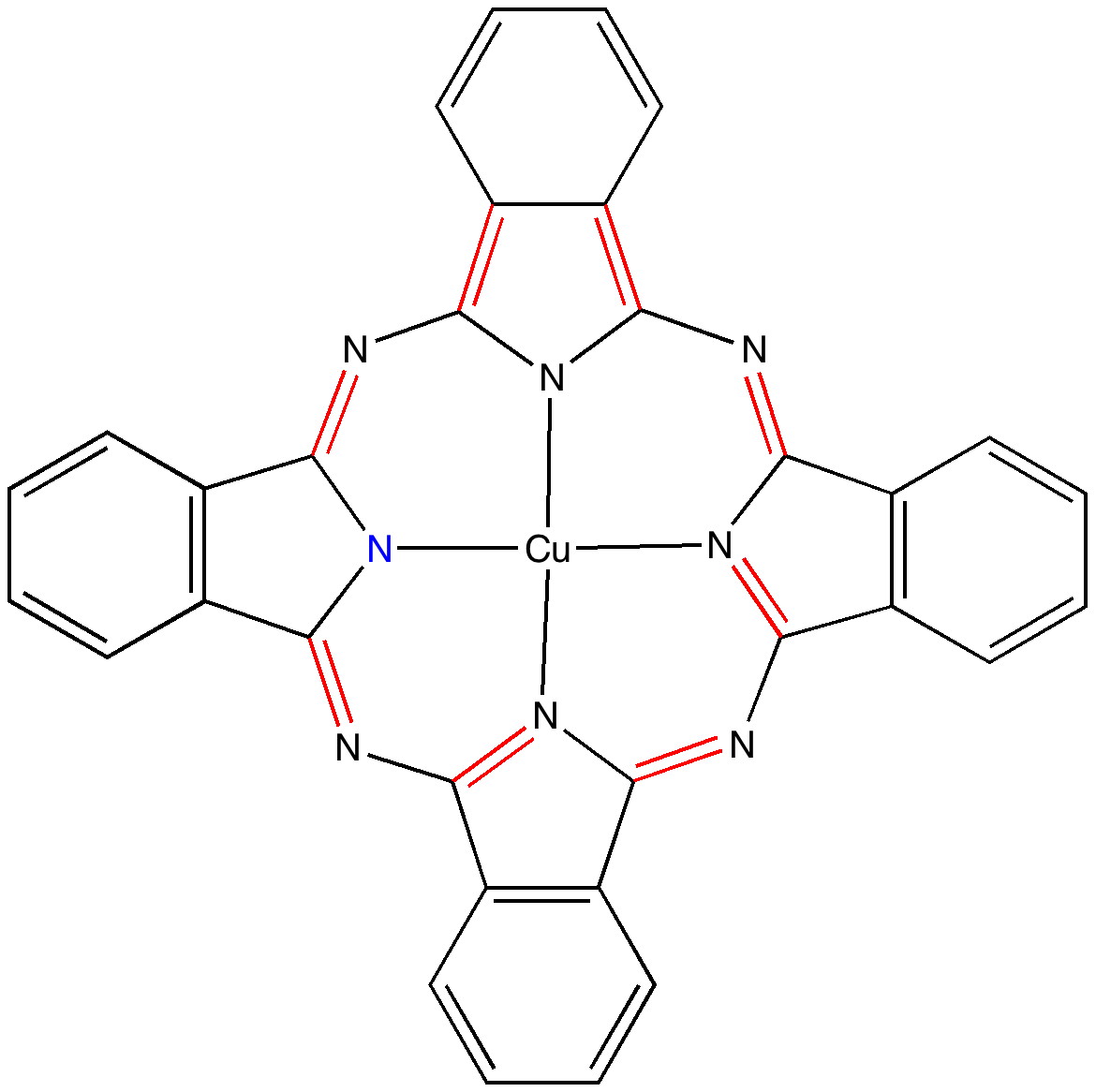
In 1933, Hückel was still thinking about his molecular orbital electronic theory of benzene, but for ~15 years, there remained little need for the rule we now know as 4n+2, because n was invariably equal to 1 for most known aromatic molecules! It was only the discovery of so-called non-benzenoid aromatics in the 1940s (e.g. Dewar’s tropolone structure) that propelled chemists to identify aromatic molecules with other values of n. And Monastral blue is a prime example of n=4 (although it would be of interest to find out when it became so associated with the Hückel rule). If you count the red bonds above, there are eight, along with one lone pair of electrons located on the highlighted (blue) nitrogen atom. This makes 18 π-electrons in the ring, or 4×4+2 (there are paths other than the one shown, but they give the same count). Part of the reason for the remarkable thermal stability of this molecule must be its aromaticity.
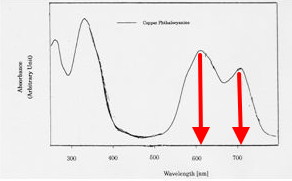
So what about the colour? The visible spectrum is shown below, with λmax ~ 610 and 710nm.
Well, a TD-DFT ωB97Xd/6-31G(d) calculation reveals the following. This reproduces the band at 610nm very nicely, but leaves the identity of the band at 710nm mysterious. How does that originate? One might speculate that this could arise from the presence of another species. Thus copper phthalocyanine itself is neutral, but it could easily be oxidised to a cation, and this could then form a 1:1 π-complex with a second molecule of the neutral radical (DOI:10.1021/ja00238a021 )
The electronic excitation at ~610nm arises from the following MOs:
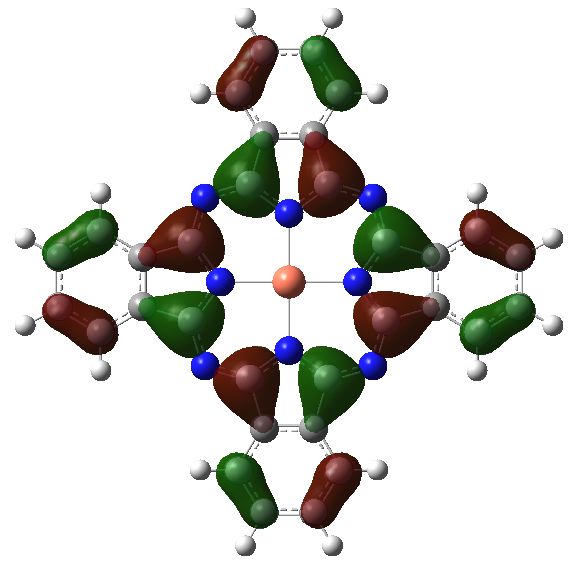 Orbital 147, the highest occupied MO (HOMO). Click for 3D |
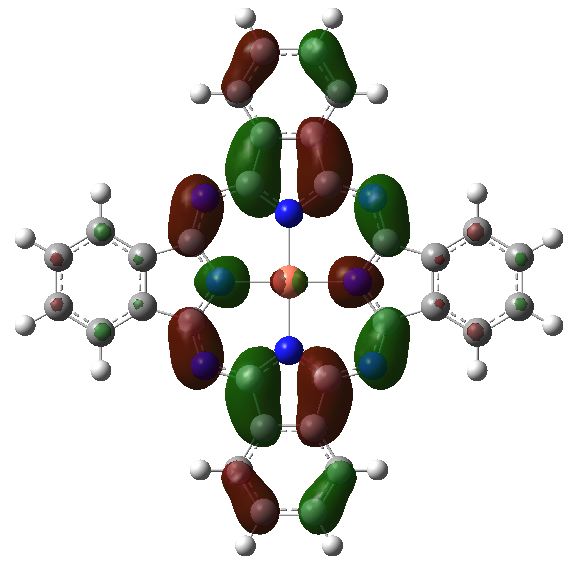 Orbital 148, the lowest unoccupied MO. Click for 3D |
The unpaired electron in copper phthalocyanine occupies the following rather interesting orbital, which appears not to be involved in its blue colour.
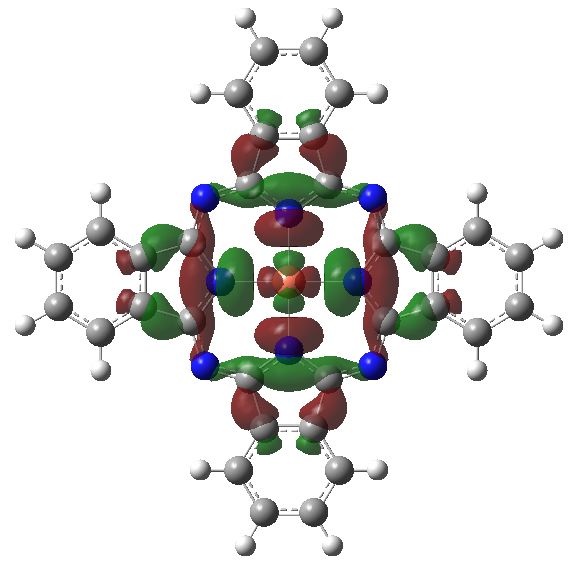
Orbital 146. The singly occupied MO. Click for 3D
So, just as with mauveine, a mystery remains. The colour of Monastral blue is not monochromatic, in that it appears to be caused by two bands in the 600-700 region. Calculation however reveals it to have only one band at 610nm. What is the other one?
Tags: 18 electron aromaticity, chemical serendipity, Historical, HTML, HTML element, Imperial College, Missouri, Monastral blue, Patrick Linstead, phthalocyanine, Phthalocyanine Blue BN, Phthalocyanines, Pigments, rector, young chemist
[…] this post ends with a bit of a mystery. The fanciest most modern computational theory gets the colour of mauveine wrong by ~100nm. […]
[…] Henry Rzepa Chemistry with a twist « Monastral: the colour of blue […]
This is the predicted Visible spectrum of the cation. λmax is ~410nm. This therefore does not explain the presence of a peak at ~710nm.

I have also tracked down a very recent experimental spectrum (DOI: 10.1016/j.tca.2010.01.019) which is shown below. The caption to this figure indicates it as spectrum (b) measured in solution (solid line) and as films on quartz substrate before (dashed lines) and after annealing in vacuum at 220 °C (dotted lines). This might indicate that in solution, solvent coordinates in the axial positions to the metal, and that in the solid, stacking and crystal modification is important.
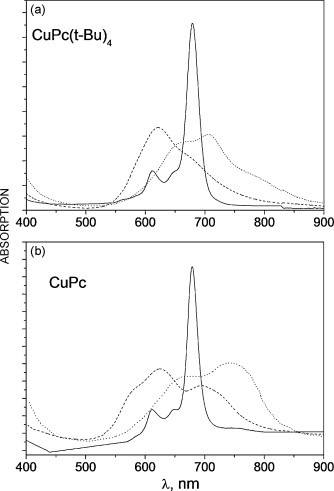
Here is the spectrum of cu-phthalocyanine.2H2O
λmax has not shifted much. Sothe 710nm peaks is not due to this.
Hello,
Many years ago i studied the photochemistry of phthalocyanines in solution… and this ‘double band’ absorption was taken as an indicator of aggregation in solution. Monomeric CuPc solutions are very difficult to work with due to its innate tendency towards aggregation…
… so i suspect that if you were to model a stacked CuPc dimer or more likely a trimer than you may be able to account for the second, longer wavelength abs band.
[…] Mclean posted a comment to my story of copper phthalocyanine (Monastral blue). The issue was its colour, and more […]