Andy Mclean posted a comment to my story of copper phthalocyanine (Monastral blue). The issue was its colour, and more specifically why this pigment has two peaks λmax 610 and 710nm making it blue. The first was accurately reproduced by calculation on the monomer, but the second was absent with such a model. Andy suggested this latter was due to stacking. Here, the calculated spectrum of a stacked dimer is explored.
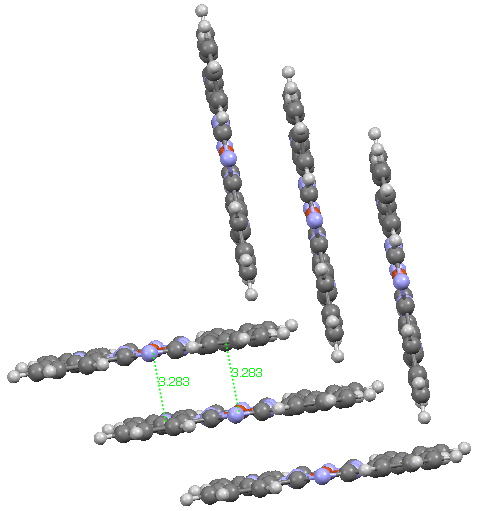
Copper phthalocyanine, showing herring-bone stacking. Click for 3D.
The hypothesis must now be that the effect of such π-π stacking on the electronic spectrum converges only slowly with the degree of stacking (if indeed it is that that is the root cause of the 710nm transition). A calculation on a triple-layered model is currently under way (this being the absolute limit of what can be done without a periodic boundary model). The spectrum will be appended to this post in a week or so (see below). There is little sign of the spectrum evolving a quite separate band at 710nm. The model is still incomplete!
Tags: Andy Mclean, copper phthalocyanine, energy, Historical, TD-DFT, X-ray