Archive for the ‘Interesting chemistry’ Category
Tuesday, June 11th, 2024
I can remember a time when journal articles carried selected data within their body as e.g. Tables, Figures or Experimental procedures, with the rest consigned to a box of paper deposited (for UK journals) at the British library. Then came ESI or electronic supporting information. Most recently, many journals are now including what is called a “Data availability” statement at the end of an article, which often just cites the ESI, but can increasingly point to so-called FAIR data. The latter is especially important in the new AI-age (“FAIR is AI-Ready”). One attribute of FAIR data is that it can be associated with a DOI in addition to that assigned to the article itself, and we have been promoting the inclusion of that Data DOI in the citation list of the article.[1] Since the data can also cite the article, a bidirectional link between data and article is established. ESI itself can exceed 1000 “pages” of a PDF document and examples of chemical FAIR data exceeding 62 Gbytes[2] (Also see DOI: 10.14469/hpc/10386) are known. Finding the chemical needle in that data haystack can become a serious problem. So here I illustrate a recent suggestion for moving to the next stage, namely the inclusion of a “Data Availability and Discovery” statement. The below is the text of such a statement in a recently published article.[3]
(more…)
References
- H. Rzepa, "The journey from Journal "ESI" to FAIR data objects: An eighteen year old (continuing) experiment.", 2023. https://doi.org/10.59350/g2p77-78m14
- T. Mies, A.J.P. White, H.S. Rzepa, L. Barluzzi, M. Devgan, R.A. Layfield, and A.G.M. Barrett, "Syntheses and Characterization of Main Group, Transition Metal, Lanthanide, and Actinide Complexes of Bidentate Acylpyrazolone Ligands", Inorganic Chemistry, vol. 62, pp. 13253-13276, 2023. https://doi.org/10.1021/acs.inorgchem.3c01506
- D.C. Braddock, S. Lee, and H.S. Rzepa, "Modelling kinetic isotope effects for Swern oxidation using DFT-based transition state theory", Digital Discovery, vol. 3, pp. 1496-1508, 2024. https://doi.org/10.1039/d3dd00246b
Posted in Interesting chemistry | 1 Comment »
Monday, February 19th, 2024
Following on from my template exploration of the Wilkinson hydrogenation catalyst, I now repeat this for the Grubbs variant of the Alkene metathesis reaction. As with the Wilkinson, here I focus on the stereochemistry of the mechanism as first suggested by Chauvin[1], an aspect lacking in eg the Wikipedia entry. As before, the diagram below is hyperlinked to the appropriate data repository identifier so that you can go straight from the scheme to the data (Top level Data DOI: [2]).
(more…)
References
- P. Jean‐Louis Hérisson, and Y. Chauvin, "Catalyse de transformation des oléfines par les complexes du tungstène. II. Télomérisation des oléfines cycliques en présence d'oléfines acycliques", Die Makromolekulare Chemie, vol. 141, pp. 161-176, 1971. https://doi.org/10.1002/macp.1971.021410112
- H. Rzepa, "Mechanistic templates computed for the Grubbs alkene-metathesis reaction.", 2024. https://doi.org/10.14469/hpc/13796
Posted in Interesting chemistry | No Comments »
Sunday, January 21st, 2024
Geoffrey Wilkinson first reported his famous work on the hydrogenation catalyst that now bears his name in 1965[1] and I met him at Imperial College around 1969 and again when I returned there in 1977. He was still working on these catalysts then and I was privileged to collaborate with him on unravelling the NMR spectra of some of these compounds.[2],[3],[4]. During that period, computational modelling of the mechanisms of molecules containing transition elements was still in its infancy and I never extended my collaboration into this area at that time. Now, even if belatedly, I decided to explore this aspect and started to do this about two weeks ago. Here I thought that I would use this opportunity to show how I am going about it.
(more…)
References
- J.F. Young, J.A. Osborn, F.H. Jardine, and G. Wilkinson, "Hydride intermediates in homogeneous hydrogenation reactions of olefins and acetylenes using rhodium catalysts", Chemical Communications (London), pp. 131, 1965. https://doi.org/10.1039/c19650000131
- K.W. Chiu, H.S. Rzepa, R.N. Sheppard, G. Wilkinson, and W. Wong, "Two-dimensional δ/J-resolved<sup>31</sup>P n.m.r. spectroscopy of [bis(diphenylphosphino)methane](trimethylphosphine)chlororhodium(<scp>I</scp>)", J. Chem. Soc., Chem. Commun., pp. 482-484, 1982. https://doi.org/10.1039/c39820000482
- C. Kwok W., C.G. Howard, H.S. Rzepa, R.N. Sheppard, G. Wilkinson, A.M. Galas, and M.B. Hursthouse, "Trimethyl and diethylphenylphosphine complexes of rhenium(I, III, IV, V) and their reactions. X-ray crystal structures of a bis(η5-cyclopentadienyl)-ethane-bridged dirhenium(I) complex obtained from phenylacetylene, tetrakis-(diethylphenylphosphine) (dinitrogen) hydridorhenium (I), tetrakis(trimethyl-phosphine) (η2-dimethylphosphinomethyl) rhenium(I) and tetrakis(trimethylphosphine) (iodo)methyl rhenium(III) iodide-tetramethylphosphonium iodide", Polyhedron, vol. 1, pp. 441-451, 1982. https://doi.org/10.1016/s0277-5387(00)86558-4
- K.W. Chiu, H.S. Rzepa, R.N. Sheppard, G. Wilkinson, and W. Wong, "Bis(diphenylphosphino)methane trimethylphosphine alkyl and η5-cyclopentadienyl compounds of rhodium(I); 31P{1H} two dimensional δ/J resolved and Overhauser effect nuclear magnetic resonance spectroscopy", Polyhedron, vol. 1, pp. 809-817, 1982. https://doi.org/10.1016/0277-5387(82)80008-9
Posted in Interesting chemistry | 2 Comments »
Thursday, May 19th, 2022
Previously, I explored the unusual structure of a molecule with a hydrogen bonded interaction between a phenol and a pyridine. The crystal structure name was RAKQOJ and it had been reported as having almost symmetrical N…H…O hydrogen bonds. This feature had been determined using neutron diffraction crystallography, which is thought very reliable at determining proton positions. Another compound with these characteristics is 3-methyl-5-phenylpyrazole or MEPHPY01.[1] Here the neutron study showed it to apparently have the structure represented below, where the solid N-H lines indicate a proton equidistant between two nitrogens.
(more…)
References
- F.H. Moore, A.H. White, and A.C. Willis, "3-Methyl-5-phenylpyrazole: a neutron diffraction study", Journal of the Chemical Society, Perkin Transactions 2, pp. 1068, 1975. https://doi.org/10.1039/p29750001068
Posted in Interesting chemistry | No Comments »
Thursday, April 7th, 2022
The previous examples of four atom systems displaying two layers of aromaticity illustrated how 4 (B4), 8 (C4) and 12 (N4) valence electrons were partitioned into 4n+2 manifolds (respectively 2+2, 6+2 and 6+6). The triplet state molecule B2C2 with 6 electrons partitioned into 2π and 4σ electrons, with the latter following Baird’s aromaticity rule.[1],[2]. Now for the final missing entry; as a triplet C2N2 has 10 electrons, which now partition into 4 + 6. But would that be 4π + 6σ or 4σ + 6π? Well, in a way neither! Read on.
(more…)
References
- N.C. Baird, "Quantum organic photochemistry. II. Resonance and aromaticity in the lowest 3.pi..pi.* state of cyclic hydrocarbons", Journal of the American Chemical Society, vol. 94, pp. 4941-4948, 1972. https://doi.org/10.1021/ja00769a025
- M. Rosenberg, C. Dahlstrand, K. Kilså, and H. Ottosson, "Excited State Aromaticity and Antiaromaticity: Opportunities for Photophysical and Photochemical Rationalizations", Chemical Reviews, vol. 114, pp. 5379-5425, 2014. https://doi.org/10.1021/cr300471v
Posted in Interesting chemistry | No Comments »
Thursday, March 24th, 2022
The meeting covered the scientific life of Professor Sir Geoffrey Wilkinson from the perspective of collaborators, friends and family and celebrated three anniversaries, the centenary of his birth (2021), the half-century anniversary of the Nobel prize (2023) and 70 years almost to the day (1 April) since the publication of the seminal article on Ferrocene (2022).[1]
(more…)
References
- G. Wilkinson, M. Rosenblum, M.C. Whiting, and R.B. Woodward, "THE STRUCTURE OF IRON BIS-CYCLOPENTADIENYL", Journal of the American Chemical Society, vol. 74, pp. 2125-2126, 1952. https://doi.org/10.1021/ja01128a527
Posted in Historical, Interesting chemistry | No Comments »
Tuesday, March 22nd, 2022
Normally, aromaticity is qualitatively assessed using an electron counting rule for cyclic conjugated rings. The best known is the Hückel 4n+2 rule (n=0,1, etc) for inferring diatropic aromatic ring currents in singlet-state π-conjugated cyclic molecules‡ and a counter 4n rule which infers an antiaromatic paratropic ring current for the system. Some complex rings can sustain both types of ring currents in concentric rings or regions within the molecule, i.e. both diatropic and paratropic regions. Open shell (triplet state) molecules have their own rule; this time the molecule has a diatropic ring current if it follows a 4n rule, often called Baird’s rule. But has a molecule which simultaneously follows both Hückel’s AND Baird’s rule ever been suggested? Well, here is one, as indeed I promised in the previous post.
(more…)
Posted in Interesting chemistry | 2 Comments »
Saturday, March 19th, 2022
I discussed in the previous post the small molecule C4 and how of the sixteen valence electrons, eight were left over after forming C-C σ-bonds which partitioned into six σ and two π. So now to consider B4. This has four electrons less, and now the partitioning is two σ and two π (CCSD(T)/Def2-TZVPPD calculation, FAIR DOI: 10.14469/hpc/10157). Again both these sets fit the Hückel 4n+2 rule (n=0).
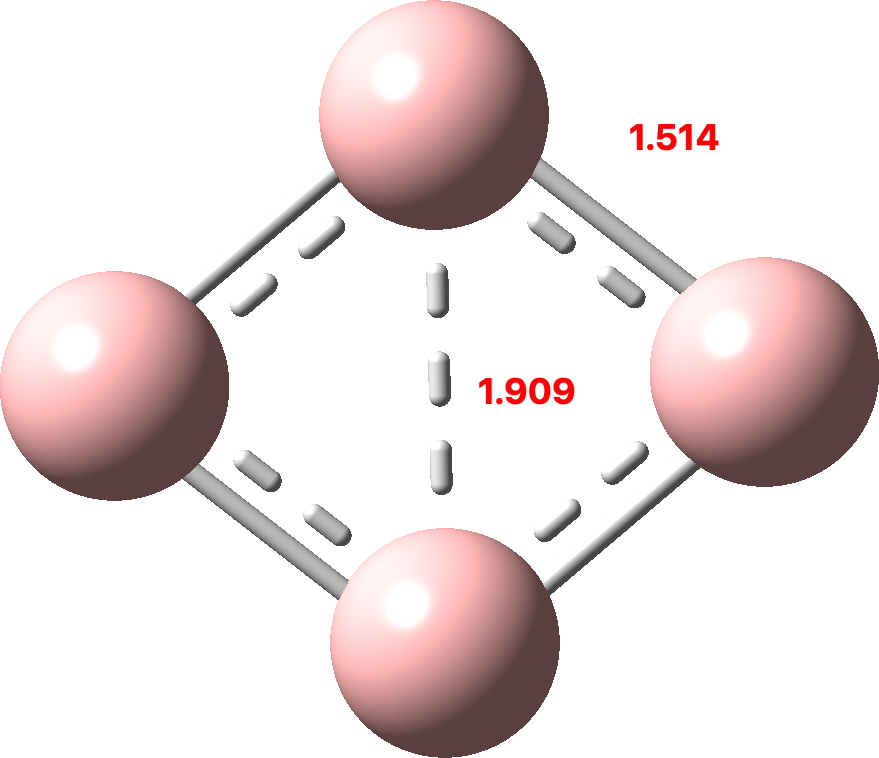 (more…)
(more…)
Posted in Interesting chemistry | No Comments »
Thursday, December 16th, 2021
The annual “molecule of the year” results for 2021 are now available … and the winner is Infinitene.[1],[2] This is a benzocirculene in the form of a figure eight loop (the infinity symbol), a shape which is also called a lemniscate [3] after the mathematical (2D) function due to Bernoulli. The most common class of molecule which exhibits this (well known) motif are hexaphyrins (hexaporphyrins; porphyrin is a tetraphyrin)[4],[5],[6], many of which exhibit lemniscular topology as determined from a crystal structure. Straightforward annulenes have also been noted to display this[7] (as first suggested here for a [14]annulene[8]) and other molecules show higher-order Möbius forms such as trefoil knots.[9],[10] This new example uses twelve benzo groups instead of six porphyrin units to construct the lemniscate. So the motif is not new, but this is the first time it has been constructed purely from benzene rings. (more…)
References
- K. Itami, M. Krzeszewski, and H. Ito, "Infinitene: A Helically Twisted Figure-Eight [12]Circulene Topoisomer", 2021. https://doi.org/10.26434/chemrxiv-2021-pcwcc
- M. Krzeszewski, H. Ito, and K. Itami, "Infinitene: A Helically Twisted Figure-Eight [12]Circulene Topoisomer", Journal of the American Chemical Society, vol. 144, pp. 862-871, 2021. https://doi.org/10.1021/jacs.1c10807
- C.S.M. Allan, and H.S. Rzepa, "Chiral Aromaticities. AIM and ELF Critical Point and NICS Magnetic Analyses of Möbius-Type Aromaticity and Homoaromaticity in Lemniscular Annulenes and Hexaphyrins", The Journal of Organic Chemistry, vol. 73, pp. 6615-6622, 2008. https://doi.org/10.1021/jo801022b
- H. Rath, J. Sankar, V. PrabhuRaja, T.K. ChandrashekarPresent address: The D, B.S. Joshi, and R. Roy, "Figure-eight aromatic core-modified octaphyrins with six meso links: syntheses and structural characterization", Chemical Communications, pp. 3343, 2005. https://doi.org/10.1039/b502327k
- H. Rath, J. Sankar, V. PrabhuRaja, T.K. Chandrashekar, and B.S. Joshi, "Aromatic Core-Modified Twisted Heptaphyrins[1.1.1.1.1.1.0]: Syntheses and Structural Characterization", Organic Letters, vol. 7, pp. 5445-5448, 2005. https://doi.org/10.1021/ol0521937
- S. Shimizu, N. Aratani, and A. Osuka, "<i>meso</i>‐Trifluoromethyl‐Substituted Expanded Porphyrins", Chemistry – A European Journal, vol. 12, pp. 4909-4918, 2006. https://doi.org/10.1002/chem.200600158
- T. Perera, F.R. Fronczek, and S.F. Watkins, "2,9,16,23-Tetrakis(1-methylethyl)-5,6,11,12,13,14,19,20,25,26,27,28-dodecadehydrotetrabenzo[<i>a</i>,<i>e</i>,<i>k</i>,<i>o</i>]cycloeicosene", Acta Crystallographica Section E Structure Reports Online, vol. 67, pp. o3493-o3493, 2011. https://doi.org/10.1107/s1600536811048604
- H.S. Rzepa, "A Double-Twist Möbius-Aromatic Conformation of [14]Annulene", Organic Letters, vol. 7, pp. 4637-4639, 2005. https://doi.org/10.1021/ol0518333
- G.R. Schaller, F. Topić, K. Rissanen, Y. Okamoto, J. Shen, and R. Herges, "Design and synthesis of the first triply twisted Möbius annulene", Nature Chemistry, vol. 6, pp. 608-613, 2014. https://doi.org/10.1038/nchem.1955
- S.M. Bachrach, and H.S. Rzepa, "Cycloparaphenylene Möbius trefoils", Chemical Communications, vol. 56, pp. 13567-13570, 2020. https://doi.org/10.1039/d0cc04190d
Posted in Chiroptics, Interesting chemistry | 5 Comments »
