Posts Tagged ‘Steve Bachrach’
Thursday, May 2nd, 2019
Ken Houk’s group has recently published this study of cycloaddition reactions, using a combination of classical transition state location followed by molecular dynamics trajectory calculations,[1] and to which Steve Bachrach’s blog alerted me. The reaction struck me as being quite polar (with cyano groups) and so I took a look at the article to see what both the original[2] experimental conditions were and how the new simulations compared. The reaction itself is shown below.
(more…)
References
- X. Xue, C.S. Jamieson, M. Garcia-Borràs, X. Dong, Z. Yang, and K.N. Houk, "Ambimodal Trispericyclic Transition State and Dynamic Control of Periselectivity", Journal of the American Chemical Society, vol. 141, pp. 1217-1221, 2019. https://doi.org/10.1021/jacs.8b12674
- C.Y. Liu, and S.T. Ding, "Cycloadditions of electron-deficient 8,8-disubstituted heptafulvenes to electron-rich 6,6-disubstituted fulvenes", The Journal of Organic Chemistry, vol. 57, pp. 4539-4544, 1992. https://doi.org/10.1021/jo00042a039
Tags:Chemistry, computational chemistry, Implicit solvation, Ken Houk, Molecular dynamics, Molecular modelling, Natural sciences, Physical sciences, Solutions, Solvent, Solvent model, Solvents, Steve Bachrach, Theoretical chemistry
Posted in reaction mechanism | 2 Comments »
Monday, June 26th, 2017
About 18 months ago, there was much discussion on this blog about a system reported by Bob Pascal and co-workers containing a short H…H contact of ~1.5Å[1]. In this system, the hydrogens were both attached to Si as Si-H…H-Si and compressed together by rings. Now a new report[2] and commented upon by Steve Bachrach, claims a similar distance for hydrogens attached to carbon, i.e. C-H…H-C, but without the ring compression.
(more…)
References
- J. Zong, J.T. Mague, and R.A. Pascal, "Exceptional Steric Congestion in an <i>in</i>,<i>in</i>-Bis(hydrosilane)", Journal of the American Chemical Society, vol. 135, pp. 13235-13237, 2013. https://doi.org/10.1021/ja407398w
- S. Rösel, H. Quanz, C. Logemann, J. Becker, E. Mossou, L. Cañadillas-Delgado, E. Caldeweyher, S. Grimme, and P.R. Schreiner, "London Dispersion Enables the Shortest Intermolecular Hydrocarbon H···H Contact", Journal of the American Chemical Society, vol. 139, pp. 7428-7431, 2017. https://doi.org/10.1021/jacs.7b01879
Tags:10.1021, Blog, chemical shift, chemical shift difference, chemical shifts, gas phase, Oxygen, Steve Bachrach
Posted in Interesting chemistry | 3 Comments »
Friday, October 23rd, 2015
Steve Bachrach on his own blog has commented on a recent article[1] discussing the structure of the trimer of fluoroethanol. Rather than the expected triangular form with three OH—O hydrogen bonds, the lowest energy form only had two such bonds, but it matched the microwave data much better. Here I explore this a bit more.
(more…)
References
- J. Thomas, X. Liu, W. Jäger, and Y. Xu, "Unusual H‐Bond Topology and Bifurcated H‐bonds in the 2‐Fluoroethanol Trimer", Angewandte Chemie International Edition, vol. 54, pp. 11711-11715, 2015. https://doi.org/10.1002/anie.201505934
Tags:Atoms in molecules, chemical bonding, Diagram, Hydrogen bond, low energy structure, lowest energy form, microwave, Steve Bachrach
Posted in Interesting chemistry | 4 Comments »
Thursday, November 6th, 2014
Solvolytic mechanisms are amongst the oldest studied, but reproducing their characteristics using computational methods has been a challenging business. This post was inspired by reading Steve Bachrach’s post, itself alluding to this aspect in the title “Computationally handling ion pairs”. It references this recent article on the topic[1] in which the point is made that reproducing the features of both contact and solvent-separated ion pairs needs a model comprising discrete solvent molecules (in this case four dichloromethane units) along with a continuum model.
(more…)
References
- T. Hosoya, T. Takano, P. Kosma, and T. Rosenau, "Theoretical Foundation for the Presence of Oxacarbenium Ions in Chemical Glycoside Synthesis", The Journal of Organic Chemistry, vol. 79, pp. 7889-7894, 2014. https://doi.org/10.1021/jo501012s
Tags:10.1021, Steve Bachrach
Posted in reaction mechanism | No Comments »
Saturday, July 5th, 2014
I was lucky enough to attend the announcement made in 2012 of the discovery of the Higgs Boson. It consisted of a hour-long talk mostly about statistics, and how the particle physics community can only claim a discovery when their data has achieved a 5σ confidence level. This represents a 1 in 3.5 million probability of the result occurring by chance. I started thinking: how much chemistry is asserted at that level of confidence? Today, I read Steve Bachrach’s post on the structure of Citrinalin B and how “use of Goodman’s DP4 method indicates a 100% probability that the structure of citrinalin B is (the structure below)”. Wow, that is even higher than the physicists. Of course, 100% has been obtained by rounding 99.7 (3σ is 99.73%) or whatever (this is one number that should never be so rounded!). 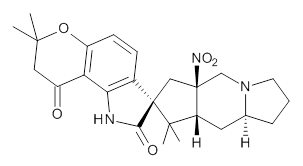 But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
(more…)
References
- E.V. Mercado-Marin, P. Garcia-Reynaga, S. Romminger, E.F. Pimenta, D.K. Romney, M.W. Lodewyk, D.E. Williams, R.J. Andersen, S.J. Miller, D.J. Tantillo, R.G.S. Berlinck, and R. Sarpong, "Total synthesis and isolation of citrinalin and cyclopiamine congeners", Nature, vol. 509, pp. 318-324, 2014. https://doi.org/10.1038/nature13273
- T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao, and J. Kobayashi, "Absolute Stereochemistry of Citrinadins A and B from Marine-Derived Fungus", The Journal of Organic Chemistry, vol. 70, pp. 9430-9435, 2005. https://doi.org/10.1021/jo051499o
- F. Cherblanc, Y. Lo, E. De Gussem, L. Alcazar‐Fuoli, E. Bignell, Y. He, N. Chapman‐Rothe, P. Bultinck, W.A. Herrebout, R. Brown, H.S. Rzepa, and M.J. Fuchter, "On the Determination of the Stereochemistry of Semisynthetic Natural Product Analogues using Chiroptical Spectroscopy: Desulfurization of Epidithiodioxopiperazine Fungal Metabolites", Chemistry – A European Journal, vol. 17, pp. 11868-11875, 2011. https://doi.org/10.1002/chem.201101129
- F.L. Cherblanc, Y. Lo, W.A. Herrebout, P. Bultinck, H.S. Rzepa, and M.J. Fuchter, "Mechanistic and Chiroptical Studies on the Desulfurization of Epidithiodioxopiperazines Reveal Universal Retention of Configuration at the Bridgehead Carbon Atoms", The Journal of Organic Chemistry, vol. 78, pp. 11646-11655, 2013. https://doi.org/10.1021/jo401316a
Tags:chemical, Reading, Steve Bachrach, X-ray
Posted in General | 2 Comments »
Friday, June 6th, 2014
Following the discussion here of Kekulé’s suggestion of what we now call a vibrational mode (and which in fact now bears his name), I thought I might apply the concept to a recent molecule known as [2.2]paracyclophane. The idea was sparked by Steve Bachrach’s latest post, where the “zero-point” structure of the molecule has recently been clarified as having D2 symmetry.[1]
(more…)
References
- H. Wolf, D. Leusser, M. Jørgensen, R. Herbst‐Irmer, Y. Chen, E. Scheidt, W. Scherer, B.B. Iversen, and D. Stalke, "Phase Transition of [2,2]‐Paracyclophane – An End to an Apparently Endless Story", Chemistry – A European Journal, vol. 20, pp. 7048-7053, 2014. https://doi.org/10.1002/chem.201304972
Tags:10.1002, 201304972, Hiberty and co, Steve Bachrach
Posted in Historical, Interesting chemistry | No Comments »
Wednesday, November 13th, 2013
Not long ago, I described a cyclic carbene in which elevating the carbene lone pair into a π-system transformed it from a formally 4n-antiaromatic π-cycle into a 4n+2 aromatic π-cycle. From an entirely different area of chemistry, another example of this behaviour emerges; Schreiner’s[1] trapping and reactions of t-butyl-hydroxycarbene, as described on Steve Bachrach’s blog. A point I often make is that chemistry is all about connections, and so here I will discuss such a connection.
(more…)
References
- D. Ley, D. Gerbig, and P.R. Schreiner, "Tunneling control of chemical reactions: C–H insertion versus H-tunneling in tert-butylhydroxycarbene", Chem. Sci., vol. 4, pp. 677-684, 2013. https://doi.org/10.1039/c2sc21555a
Tags:Steve Bachrach
Posted in pericyclic, reaction mechanism | No Comments »
Sunday, October 20th, 2013
Homoaromaticity is a special case of aromaticity in which π-conjugation is interrupted by a single sp3 hybridized carbon atom (it is sometimes referred to as a suspended π-bond with no underlying σ-foundation). But consider the carbene shown below. This example comes from a recently published article[1] which was highlighted on Steve Bachrach’s blog. Here aromaticity has resulted from a slightly different phenomenon, whereby a 4π-electron planar (and hence nominally anti-aromatic) molecule is elevated to aromatic peerage by promoting the two carbene σ-electrons to have π-status.
(more…)
References
- B. Chen, A.Y. Rogachev, D.A. Hrovat, R. Hoffmann, and W.T. Borden, "How to Make the σ<sup>0</sup>π<sup>2</sup> Singlet the Ground State of Carbenes", Journal of the American Chemical Society, vol. 135, pp. 13954-13964, 2013. https://doi.org/10.1021/ja407116e
Tags:low energy, Steve Bachrach
Posted in Interesting chemistry | 1 Comment »
 But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes