The previous post contained an exploration of the anomeric effect as it occurs at an atom centre X for which the effect is manifest in crystal structures. Here I quantify the effect, by selecting the test molecule MeO-X-OMe, where X is of two types:
Posts Tagged ‘interaction energy’
A periodic table for anomeric centres, this time with quantified interactions.
Monday, August 8th, 2016A periodic table for anomeric centres.
Saturday, August 6th, 2016In the last few posts, I have explored the anomeric effect as it occurs at an atom centre X. Here I try to summarise the atoms for which the effect is manifest in crystal structures.
The conformational preference of s-cis amides.
Sunday, February 10th, 2013Amides with an H-N group are a component of the peptide linkage (O=C-NH). Here I ask what the conformation (it could also be called a configuration) about the C-N bond is. A search of the following type can be defined:
Hydrogen bond strength as a function of ring size.
Thursday, January 3rd, 2013One frequently has to confront the question: will a hydrogen bond form between a suitable donor (lone pair or π) and an acceptor? One of the factors to be taken into consideration for hydrogen bonds which are part of a cycle is the ring size. Here I explore one way of quantifying the effect for the series below, n=1-5 (4-8 membered rings).
Spotting the unexpected. The trifluoromeric effect in the hydration of the carbonyl group.
Friday, March 9th, 2012The equilibrium for the hydration of a ketone to form a gem-diol hydrate is known to be highly sensitive to substituents. Consider the two equilibria:
A comparison of left and right handed DNA double-helix models.
Saturday, January 1st, 2011When Watson and Crick (WC) constructed their famous 3D model for DNA, they had to decide whether to make the double helix left or right handed. They chose a right-handed turn, on the grounds that their attempts at left-handed models all “violated permissible van der Waals contacts“. No details of what these might have been were given in their original full article (or the particular base-pairs which led to the observation). This follow-up to my earlier post explores this aspect, using a computer model.
The mysteries of stereoinduction.
Thursday, July 1st, 2010Stereo-induction is, lets face it, a subtle phenomenon. The ratio of two stereoisomers formed in a reaction can be detected very accurately by experiment, and when converted to a free energy difference using ΔG = -RT Ln K, this can amount to quite a small value (between 0.5 – 1.5 kcal/mol). Can modelling reproduce effects originating from such small energy differences? Well one system that has been argued about now for several decades is shown below as 1.
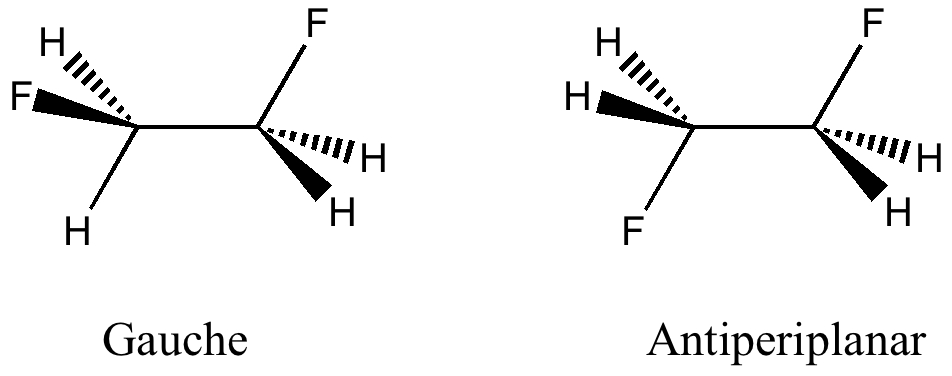
The conformation of 1,2-difluoroethane
Tuesday, April 6th, 2010Here I offer another spin-off from writing a lecture course on conformational analysis. This is the famous example of why 1,2-difluoroethane adopts a gauche rather than antiperiplanar conformation.
How do molecules interact with each other?
Sunday, April 12th, 2009Understanding how molecules interact (bind) with each other when in close proximity is essential in all areas of chemistry. One specific example of this need is for the molecule shown below.