Alkene metathesis is part of a new generation of synthetic reaction in which a double C=C bond is formed from appropriate reactants where no bond initially exists (another example is the Wittig reaction), with the involvement† of a 4-membered-ring metallacyclobutane ring 1 (again, very similar to the Wittig). I thought it might make a good addition to my collection of reaction mechanisms and so as the first step I set about locating the transition state (TS or TS’) for the reaction, using in this case a model for Grubbs’ catalyst. I have located a fair few transition states in my time, and was frankly not expecting a surprise. This is the story that showed otherwise …
The reaction involves the formation of a C-C bond, and one can normally rely on that bond length being in the range 2.0 – 2.3Å. Thus the thermal 2+2 cycloaddition of two ethenes can have a C-C length of 2.0Å, albeit accompanied by a fascinating trapezoidal geometry. My initial thought was that this reaction might be similar. Using as the metal Ru (the one deployed for the Grubbs catalyst) the hunt proved to be unusual difficult. Eventually, it emerged (ωB97XD/Def2-SVPD/SCRF=dichloromethane) as shown below (I have deployed simple ammine ligands as replacements for the usually used pyridine, and chlorine around the metal; at this stage the subtleties of steric and electronic tuning of the catalyst are not needed).
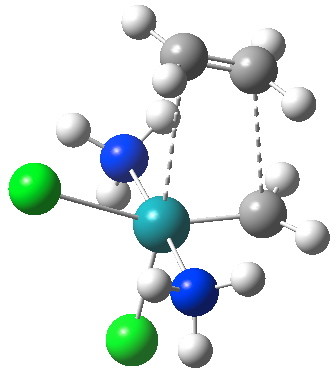
Transition state for alkene metathesis. Click for 3D.
The C-C bond is 3.0 Å, well outside the normal limit of forming C-C bonds. Indeed, at this length it has hardly started to form at all (neither has the Ru-C bond, at 3.4Å). So conventionally one would conclude it must be an early (very early) transition state, and such would also have a very small barrier to reaction (thus the barrier for cycloaddition between osmium tetroxide and propene is < 1 kcal/mol for an Os...O length of 2.36Å) But no, the IRC shows the barrier is around 14 kcal/mol (quite reasonable for a thermally facile reaction).
The IRC reveals all. Put simply, the initial Ru complex has a trigonal bipyramidal geometry. Such a shape has no free ligand site large enough to accommodate an incoming alkene. A free site can be however generated by changing the metal coordination to square pyramidal. So the initial approach of an alkene plays that role, by effectively repelling the Cl and Ru=CH2 ligands into the square pyramidal geometry. This process by the way is not dissimilar[1] to pseudorotation in PCl5. No C-C bond formation can happen whilst this geometrical reorganisation takes place (another example of high barriers induced purely by changes in bond angles is atropisomerism in taxol).
| Reorganisation of ligands up to TS | Formation of bonds after TS |
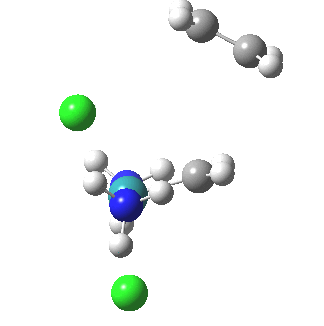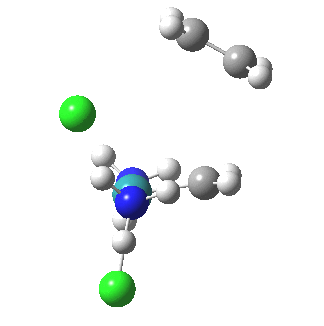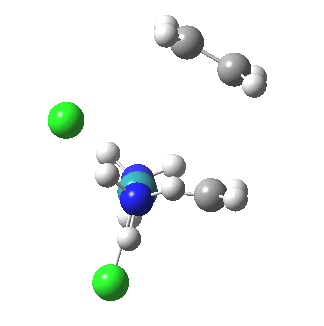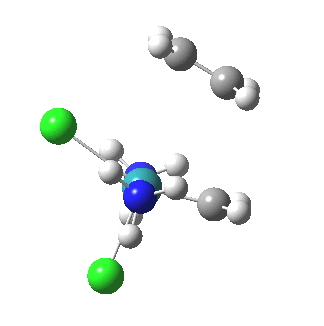 |
 |
It is only after the transition state is passed that the bond formation can start to take place. So this reaction takes place in two very distinct stages, a reorganisation of the coordination around the metal, and then bond formation. Why might this be interesting? Well, because designing a better catalyst requires knowledge of the intrinsic reorganisations involved, and the order in which they happen. One might imagine that such two-stage behaviour in catalysts is in fact not that unusual.¶
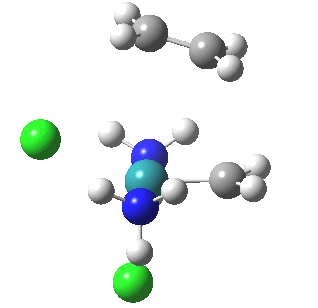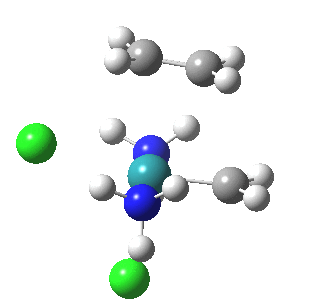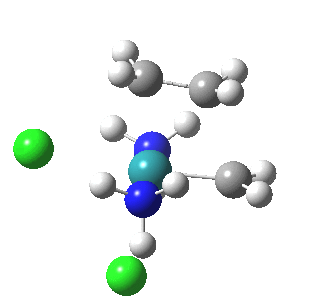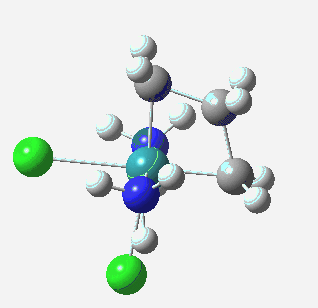
† Several ruthenium metallacyclobutanes have been isolated as crystalline solids. One example is shown below.
A ruthenium metallacyclobutane. Click for 3D.
¶ Another example is the carbonylation of methyl manganese pentacarbonyl, which I will cover in a future post.
References
- M.E. Cass, K.K. Hii, and H.S. Rzepa, "Mechanisms That Interchange Axial and Equatorial Atoms in Fluxional Processes: Illustration of the Berry Pseudorotation, the Turnstile, and the Lever Mechanisms via Animation of Transition State Normal Vibrational Modes", Journal of Chemical Education, vol. 83, pp. 336, 2006. https://doi.org/10.1021/ed083p336.2
Tags: free ligand site, metal, metal coordination, Reaction Mechanism
[…] Henry Rzepa Chemistry with a twist « Alkene metathesis springs a surprise. […]