The reaction described in the previous post (below) is an unusual example of nucleophilic attack at an sp2-carbon centre, reportedly resulting in inversion of configuration[1]. One can break it down to a sequence of up to eight individual steps, which makes teaching it far easier. But how real is that sequence?
Starting with the first of these steps, the heterolytic cleavage of the C-OTf bond to form a vinyl carbocation.The transition state, located at the ωB97XD/6-31G(d,p)/SCRF=ethanol level, reveals a more complex process in which the alkene assists in the eviction of the triflate leaving group and for which the immediate product is the carbocation precursor of molecule B, not A.
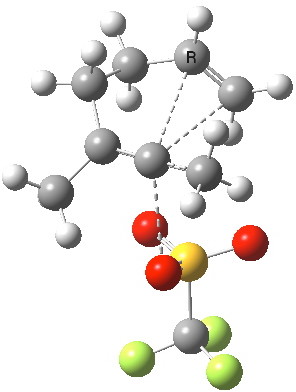 Transition state for C-OTf heterolysis. Click for 3D. |
 IRC for C-OTf heterolysis. Click for final product. |
The IRC shows that the bonds all cleave/form at different times.
- From IRC 12-5, a conformational reorganisation occurs to prepare the alkene for assisting in the …
- C-OTf heterolysis, which is largely complete at the transition state (IRC=0.0) with a C-O bond length of 2.7Å (green arrow). The potential energy surface at the transition state is unusually flat; the imaginary reaction mode, νi has the very low value for a reaction involving cleaving bonds of 90 cm-1. The putative C-C bonds which are about to form are still very long (3.0, 3.1Å) and the transition state is quite close to instead being a vinyl carbocation intermediate. Similar behaviour was computed for the purported Sn1 solvolysis of tert-butyl chloride, in which the tertiary carbocation is not an actual intermediate on the reaction path but is instead a transition state for the reaction.
- At IRC -4, the first C-C bond is forming (blue arrow), a process largely completed by IRC -7.5.
- At IRC -10, the second C-C bond is forming (red arrow).
- The initial product of this process is the ion-pair deriving from molecule B.
I want to spend a little more time with the transition state for the substitution process described above.
- Firstly, it is worth noting that it represents inversion of configuration at the reacting sp2-hybridised carbon atom, much like that which occurs for substitution reactions of sp3-hybridized carbon.
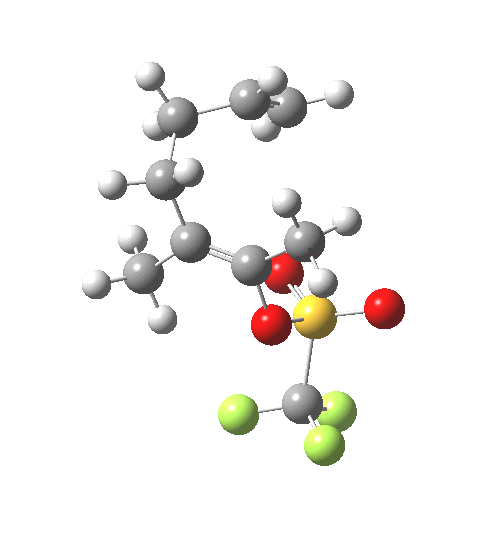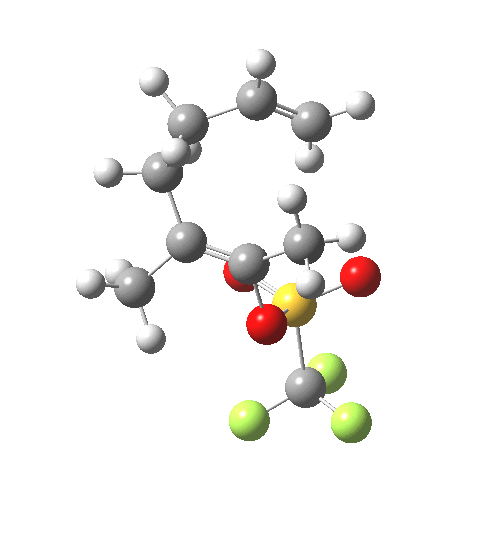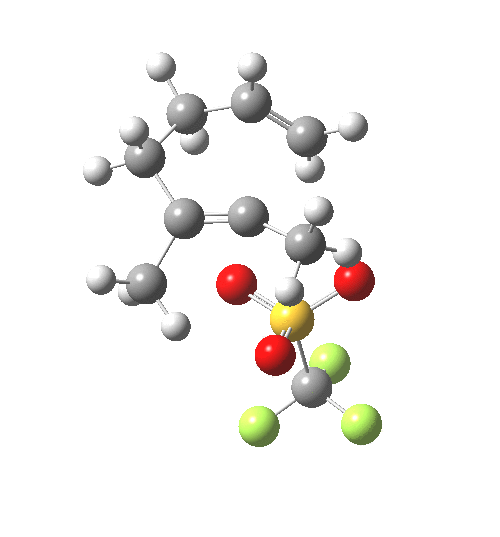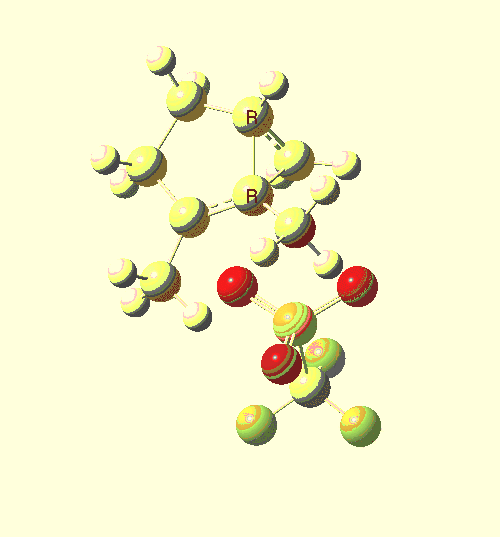
- A vinyl carbocation sounds exotic, but an iso-electronic species related to it in which boron replaces the carbon can in fact be crystallised. This makes for a neutral molecule, rather than the ion-pair involved in our reaction. An example is shown below[2]. As with our transition state, the angle subtended at the central atom is 180°. These types of system are also very reactive, which is why they can only be made if substituted with bulky groups, and one might presume that these boron analogues would also react readily with alkenes.
VARJED. Click for 3D.
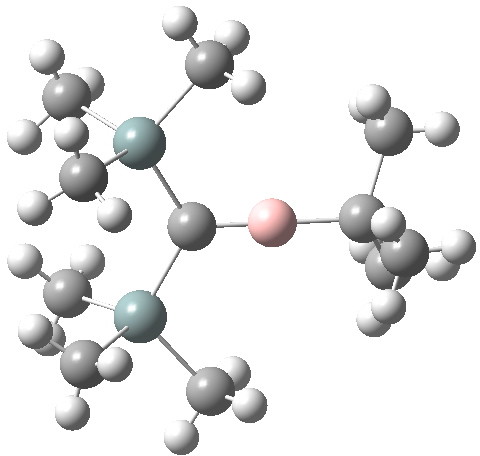
- One can go one step further and isolate a stable vinyl carbocationic ion-pair itself, as in the example shown below[3].
LOKRIN. Click for 3D.
- One can also learn from looking at the orbitals of our transition state. The NBO (natural bond orbital) shows a very prominent interaction between the alkene acting as a π-donor and the vacant p-orbital on the vinyl carbocation acting as an acceptor; almost a π-complex in fact, and on its way to making a three-membered ring (blue ≡ purple, red ≡ orange).
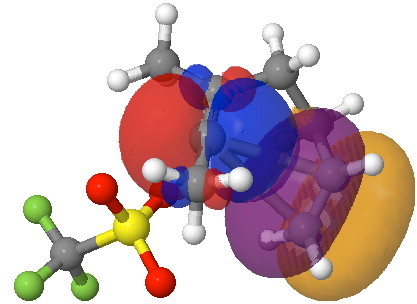
Click for rotatable orbitals.
So we have learnt that the breakdown into small steps that I used for pedagogic reasons in order to understand how the reaction proceeds is in fact conflated into one single concerted mechanism forming the product B. But we also now know that although these steps occur in concerted fashion, they do not occur in a synchronous manner. So it is still worth breaking the reaction into these steps, one must simply recognise that they can occur consecutively, and without any explicit intermediates involved.
I will take a break here, and deal with the formation of the other product, molecule A, in the next post.
References
- T.C. Clarke, and R.G. Bergman, "Olefinic cyclization at a vinyl cation center. Inversion preference for intramolecular nucleophilic substitution by a double bond", Journal of the American Chemical Society, vol. 94, pp. 3627-3629, 1972. http://dx.doi.org/10.1021/ja00765a062
- R. Boese, P. Paetzold, A. Tapper, and R. Ziembinski, "Alkylalkylidenborane R BC(SiMe3)2: Isolierbare Moleküle mit zweifach koordiniertem Sextett‐Boratom", Chemische Berichte, vol. 122, pp. 1057-1060, 1989. http://dx.doi.org/10.1002/cber.19891220607
- A. Klaer, W. Saak, D. Haase, and T. Müller, "Molecular Structure of a Cyclopropyl Substituted Vinyl Cation", Journal of the American Chemical Society, vol. 130, pp. 14956-14957, 2008. http://dx.doi.org/10.1021/ja8069055
Tags: immediate product, Interesting chemistry, inversion, Reaction Mechanism, triflate leaving, Tutorial material, vinyl carbocation
[…] Henry Rzepa Chemistry with a twist « Secrets of a university tutor. An exercise in mechanistic logic: first dénouement. […]