Following on from our first mechanistic reality check, we now need to verify how product A might arise in the mechanism shown below, starting from B.
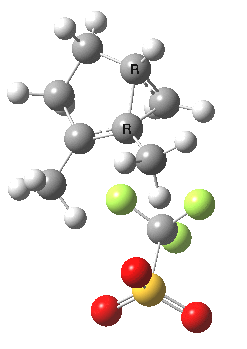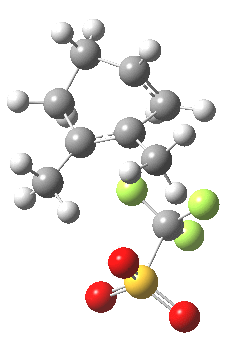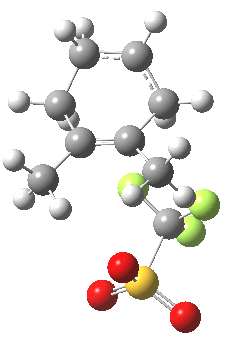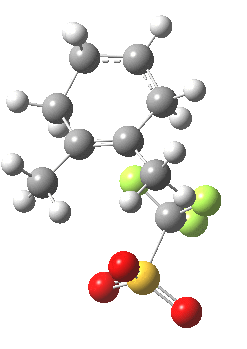
This pathway backtracks the original one in reversing the final arrow of that process (shown in red in previous post and in magenta here for the arrow in reverse), to go uphill in energy to reach the secondary (unstabilised) carbocation. This turns out to be a very shallow minimum, almost merely a ledge on the mountainside. It is not difficult to see how the original pathway down to B’ might have missed this Y-fork (= bifurcation).
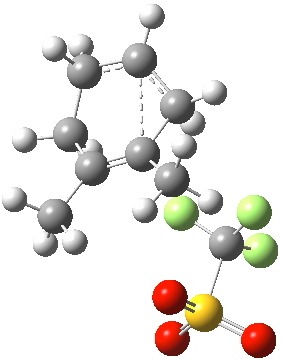 Transition state for cyclopropyl ring opening. Click for 3D. |
|
 |
|
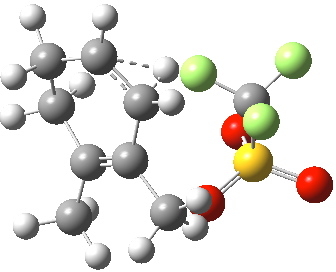
This unstable carbocation does not hang around; the barrier to transfer of a hydrogen (orange arrows) is tiny. This motion completes the formation of the product A.
 |
|
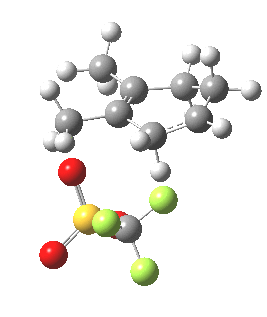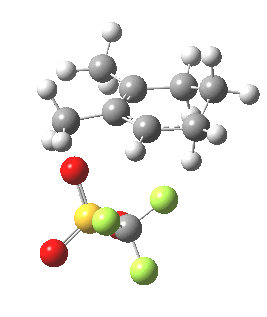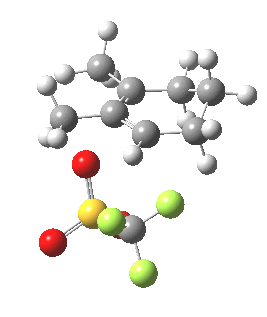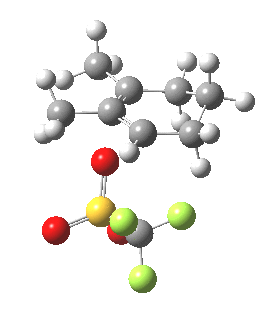 |
|
We have seen here a classical analysis of mechanism in terms of an energy profile that has a separate pathway and associated transition state for each product in a reaction. But one should note that there are increasing claims for reactions whose outcome is determined not by an explicit transition state for each reaction pathway, but where the very dynamics of the system as it exits from a single transition state can result in a bifurcation into two (or indeed more) final products. I would like to suggest that the reaction described here might also be such an example. Thus although the mechanism as shown below shows just the single product B, it might be that only a small diversion from that initial pathway would also result in formation of A, and that there would be no need for the explicit transition states to this species as shown above to be actually visited.
It would finish by noting that all the mechanisms above were studied with inclusion of the triflate counter-ion; indeed the first step could not really be studied without it. The system seems a good candidate for a thorough molecular dynamics exploration; I have certainly come a long way from an introductory tutorial in organic chemistry!
Tags: energy, energy profile, final products, Reaction Mechanism, Tutorial material