I have mentioned Lewis a number of times in these posts; his suggestion of the shared electron covalent bond still underpins much chemical thinking. Take for example mechanistic speculations on the course of a reaction, a very common indulgence in almost all articles reporting such, and largely based on informed arrow pushing. This process is bound to follow the rules of reasonable Lewis structures for any putative intermediates. Here, I suggest that we are now firmly in an era where such speculations must of necessity be backed up by quantum mechanical estimates of the energies and structures. I would propose that journals routinely encourage referees to insist on such (additional) checks. Let me give one specific example of the need to do this (part of a follow up to an earlier article I blogged on previously).
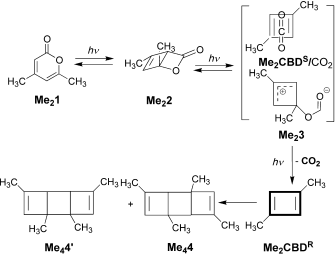
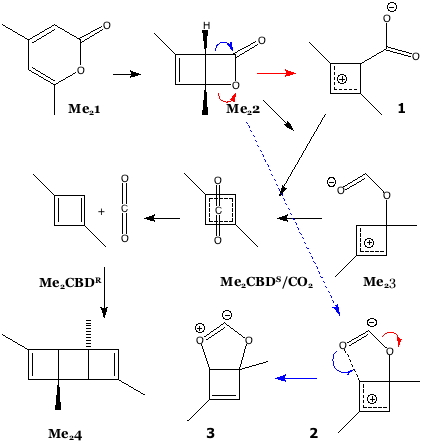
The example is found as scheme 1 of an article written by Legrand, Gilles, Petit, van der Lee and Barboiu entitled “Unprecedented Synthesis of 1,3-Dimethylcyclobutadiene in the Solid State and Aqueous Solution” (DOI: 10.1002/chem.201100693; Scheme 1 reproduced here with the permission of the publishers). Structures 1 – 3 are my additions, and are not present in scheme 1 of the above article.
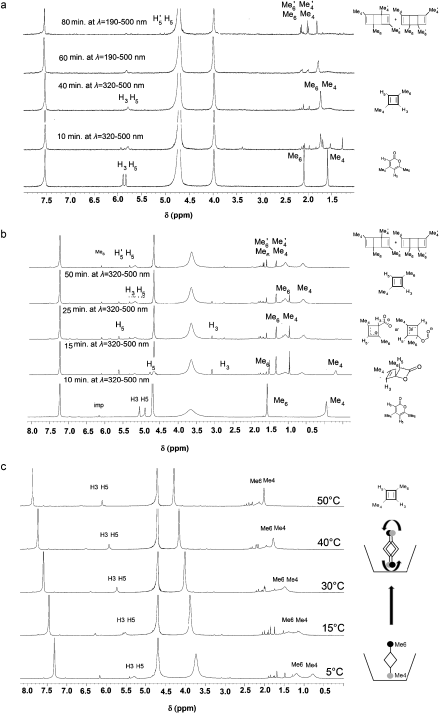
The scientific problem is to identify what the products are of photolysis of Me21. The species is contained as a guest inside a calixarene host, the whole assembly being dissolved in water (D2O). This was photolysed and the products characterised by (inter alia) their 1H NMR spectra, Figure 7. Focus in particular on 7b, which shows a set of five spectra that are claimed to identify the consecutive species Me21, Me22, (Me23 or 1), Me2CBDS/CO2, Me2CBDR and Me24 as the outcomes of photolysis at “different irradiation times at l=320–500 nm or at l=190–500 nm“.
How might one apply a computational reality check to this scheme? Lewis himself might have ventured to suggest that representation Me23 does not adhere to his rules; a modern chemistry student would draw it instead as 2, a vinyl zwitterion. This species in turn could either eliminate carbon monoxide (red arrow) or ring close to give the unusual ylid 3 (blue arrow). In fact DFT calculations on the isolated molecules in water (ωB97XD/6-311G(d,p)/SCRF=water) indicate that the C-O bond in an isolated molecule of Me23 does not persist and fragments to carbon monoxide and an alkoxy zwitterion, making it around ~36.5 kcal/mol higher in free energy than the alternative zwitterion 1. The third species 3 is somewhat more stable, being ~20 kcal/mol above 1. Calculations also reveal that whilst rectangular Me2CBDR is obtained on the singlet surface, the square Me2CBDS/CO2 can only be obtained on the triplet surface. This state however is ~8-10 kcal/mol higher in energy and unlikely to have a long lifetime before it decays down to the singlet surface. One could study all the species in the scheme above in this manner, but that analysis is for another place and time.
Until relatively recently, such reality checks would be all one might attempt computationally. But these experiments were NOT conducted on isolated molecules in solution, they were done in the presence of a calixarene host. Could that change things? Zwitterion 1 can be placed inside this cavity and the calculation repeated (again simulating solvent water), as can 2. In fact the latter spontaneously collapses to 3, and now has an energy ~ 27 kcal/mol higher than 1. Whether 1 itself (or indeed Me2CBDR) has any persistent lifetime is another issue, and one not addressed in this blog post.
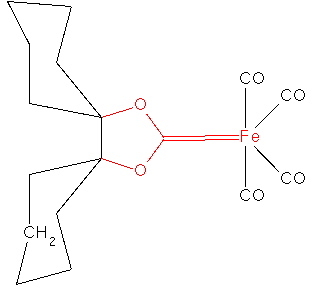
In fact, the reality check has another purpose, which is to stimulate other ideas. In this case for example one could regard 3 as a carbene, in which case one might ask if coordination of the carbene to a suitable metal might be a stabilizing mode. Amazingly, a number of such systems are known! I show just one below.
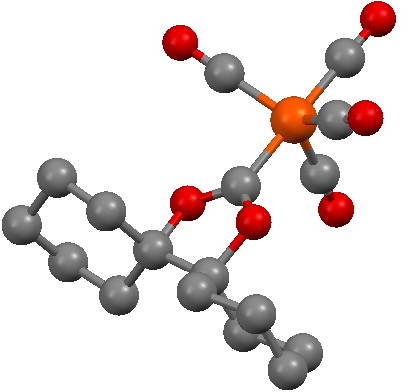 SCHXFe. Click for 3D structure. |
There is a lot more that could be said (and written) about this article, including discussion of the 1H NMR spectra, but I will stop at this point. Hopefully, I have shown how simple computational reality checks on a proposed mechanism can easily result in both unexpected outcomes and ideas for new chemistry!
Tags: 3-dimethylcyclobutadiene, blog server, calixarene, chemical thinking, energy, free energy, Lewis, pericyclic, Petit, photolysis., square Me, suitable metal
[…] Henry Rzepa Chemistry with a twist « Computational “reality checks” for mechanistic speculations. […]
[…] approach such a problem nowadays? Well, I have oft argued on this blog that a good place to obtain an immediate reality check on a proposed mechanism is a calculation. It will come as no surprise that a very accurate calculation can be done on the […]