This story starts with a calixarene, a molecule (suitably adorned with substituents) frequently used as a host to entrap a guest and perchance make the guest do something interesting. Such a calixarene was at the heart of a recent story where an attempt was made to induce it to capture cyclobutadiene in its cavity.
At the base of the calixarene are four hydroxyl groups, arranged in either a left or right handed manner. The molecule, in other words is chiral (C4 symmetry to be precise). As a chiral molecule, it might trap left and right-handed guests in a slightly different manner (forming two possible diastereomeric host-guest complexes). As it happens, the guest in the cyclobutadiene story was just such a chiral molecule. But an essential question to ask is what the barrier to enantiomerization of such a calixarene might be? One can envisage several ways of accomplishing such a conversion.
All four hydrogens can be moved in a single step, one might move two at a time in two steps, or one might move one at a time in four steps. These processes would involve respectively 8, 6 or 4 electrons in each step. There is a fundamental difference between the first pathway and the last two; the latter involve ionic intermediates (zwitterions) whereas the first is neutral. As such one might imagine the process would depend on the ability of the solvent to stabilize any such zwitterion.
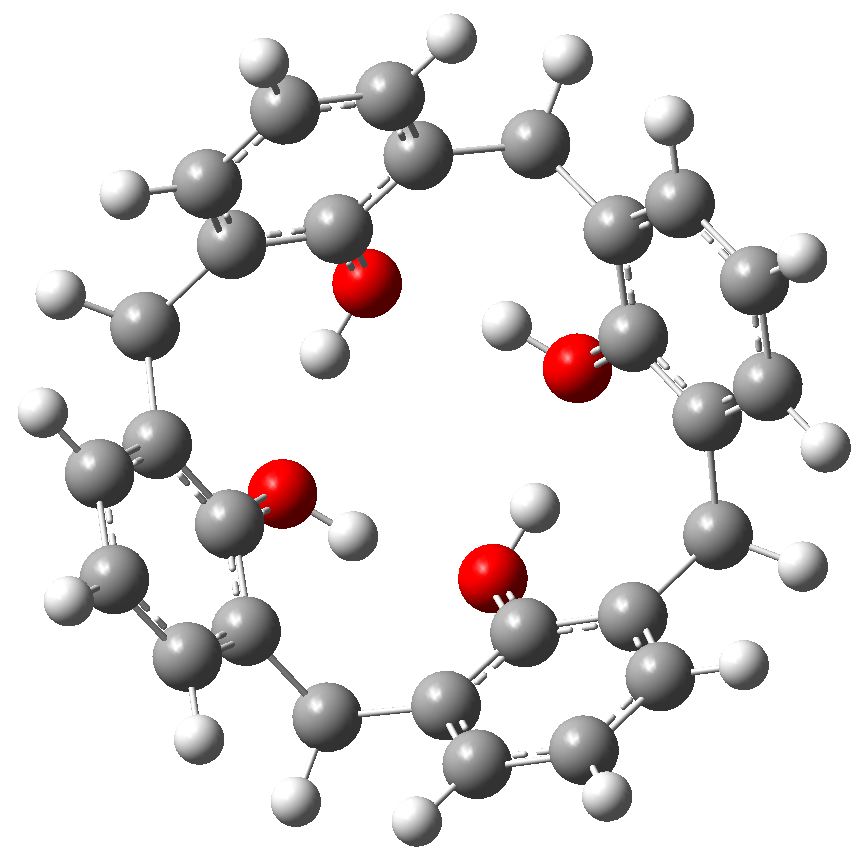
Let us start with a gas phase model (ωB97XD/6-311G(d,p)), and a transition state with one negative force constant is indeed found with C4v symmetry. The free energy barrier ΔG† for the process is 14.0 kcal/mol, which means the reaction will occur rapidly, even at lower temperatures of ~200K. A pack size of 8 seems preferred for this model. This is hardly a surprise since the formation of ionic intermediates would not be expected. One might however speculate thus. In the schematic above, n=1 and one might be tempted to ask if higher values of n (lets say n=2, a pack size of 10, or n=3, a pack size of 12, etc ) might exhibit similar behaviour. Is there any limit to the ring/pack size for this type of proton exchange?

Transition state for enantiomerization of a calixarene in the gas phase. Click for 3D.
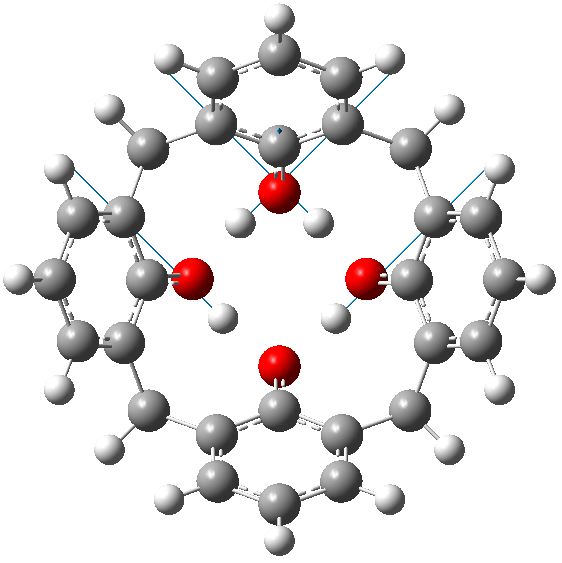 C4v symmetric geometry for calixarene in benzene solvent, with three negative force constants. Click for animation of E mode. |
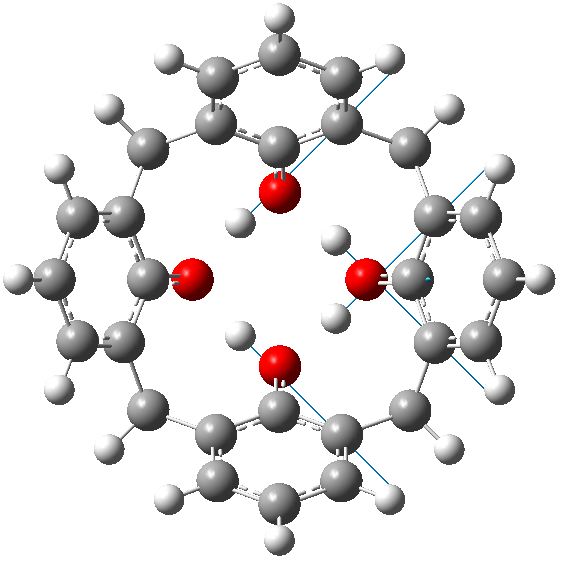 C4v geometry for calixarene in benzene. Click for animation of second E mode. |
Each of these additional two negative force constants shows a displacement heading towards the zwitterion shown in the scheme below. As one increases the polarity of the solvent, so the force constant becomes more negative. Thus for dichloromethane, it is now 322i cm-1 and with water it is 376i cm-1
So now the question is what happens when either of the two additional negative force constants is followed downhill? Will it form a true zwitterion (which would have Cs symmetry), in which case it would be (two) 6 electron processes to enantiomerize the calixarene instead of one 8 electron one.

True transition state for proton exchange in solution phase calixarene
Still unresolved is whether such cyclic transfer of four protons between four oxygen atoms continues to be concerted for larger rings, or whether the system is finally tempted to break up the transfer by resting with one or more discrete intermediates along the way. I finally note that in the calixarene reported which catalysed the thoughts above, the four oxygens are capped with a guanidinium cation sitting just above them, and this too may have an interesting effect on the proton transfer process.
Tags: animation, calixarene, chiral, dielectric, free energy barrier, gas phase, gas phase model, pericyclic, proton transfer, watoc11, zwitterionic
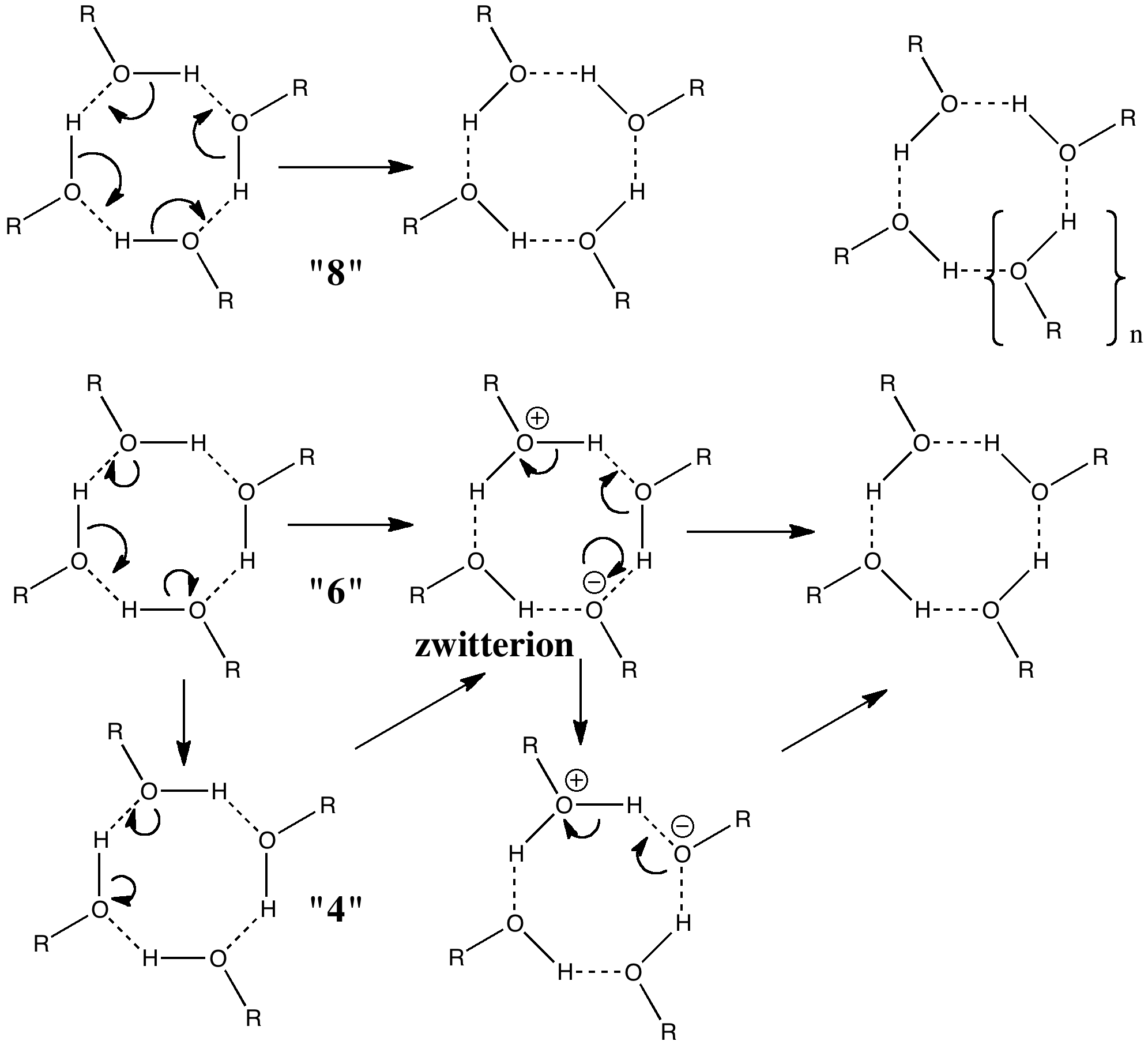
[…] issue of electron dynamics. Path (c) formally involves six electrons, path (b) only two. In a previous post, I speculated whether the electronic pack size for proton transfer was 4,6 or 8 electrons. Perhaps […]
I guess these two works are relevant to this discussion: http://www.nature.com/nature/journal/v397/n6716/full/397241a0.html (doi: 10.1038/16672 ) and http://science.sciencemag.org/content/291/5501/100 (doi: 10.1126/science.291.5501.100)