I have previously looked at the pigments used to colour the Book of Kells, which dates from around 800 AD and which contained arsenic sulfide as the yellow colourant. The Bayeaux tapestry is a later embroidery dating probably from around 1077 and here the colours are based entirely on mordanted natural dyes. These are generally acknowledged to be blue woad (principle component indigo), red madder (principle component alizarin) and the less well-known yellow weld, which comes from the plant Reseda Luteola and the principle component of which is luteolin.[1]
Luteolin has an interesting chemical history. It was first purified in 1829, in the dawn of organic chemistry, and its formula C15H10O6 established by 1864. A. G. Perkin, the son of the William Perkin who discovered the dye mauveine, then provided the chemical structure[2] in 1896. This latter article is well worth a modern read, since it beautifully illustrates how the art of structure determination was conducted in the days before crystallography and NMR.
Perkin obtains his structure by comparing luteolin to then known quercetin, concluding that the former must also contain an aromatic hydroxy group “ortho” to the carbonyl group, as in querecetin. The key experimental evidence was that alkylation of luteolin with iodoethane only produces a triethoxy derivative of luteolin, with “one hydroxy group resisting ethylation“. It was by then established, by four different sets of researchers, that hydroxy groups adjacent to the carbonyl in e.g. quercetin or alizarin resisted alkylation. The structure of luteolin was established (see eg 10.5517/cc798yq) by combining various such observations, a method (and skill) that has largely lapsed nowadays.
A modern take on this selective alkylation might be to compute e.g. the wavefunction (ωB97XD/Def2-TZVPP/SCRF=water) of luteolin to inspect the energies of the orbitals associated with alkylation of the hydroxyl group, using the energy of the nucleophilic lone pair oxygen orbital (FAIR DOI: 10.14469/hpc/12185) as an indicator. The least stable such orbital (highest energy) is normally an indicator of the most nucleophilic electron pair. In this case, the highest (most reactive) such orbital is the one adjacent to the carbonyl group, which thereby reveals a mystery, since it is this very hydroxyl that resists alkylation! A transition state approach to this might be needed to resolve the mystery, factoring in perhaps steric effects etc.
| -0.6951 au | -0.7132 au |
|---|---|
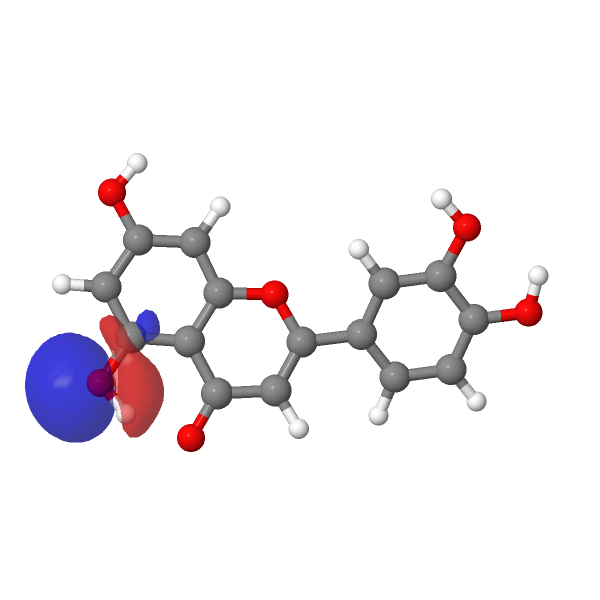 |
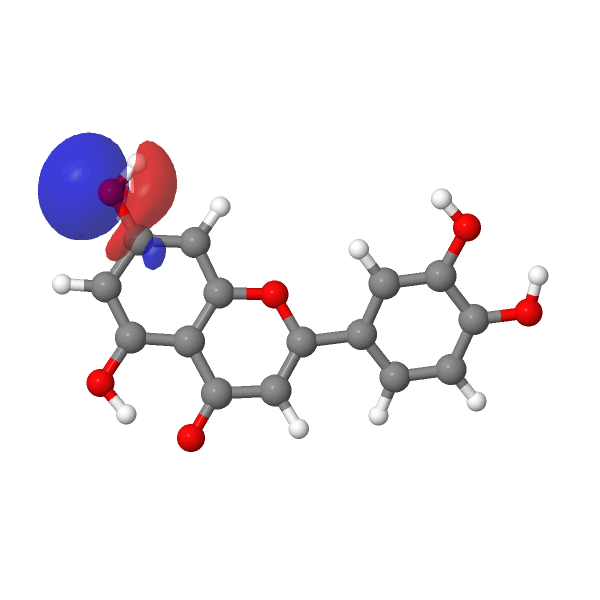 |
| -0.7169 au | -0.7205 au |
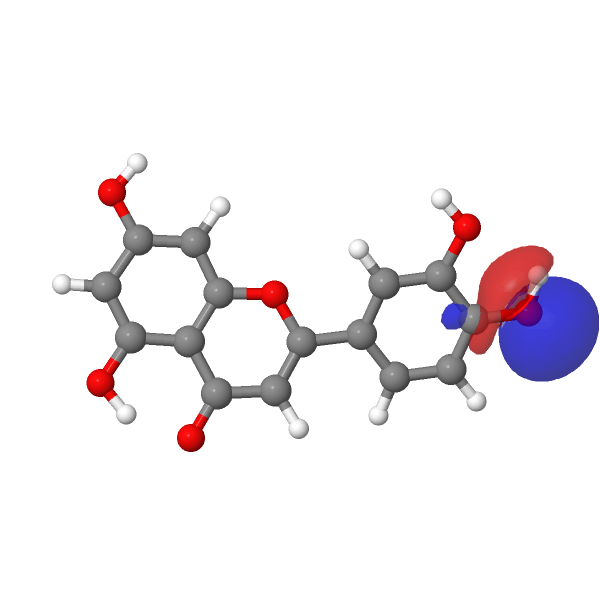 |
<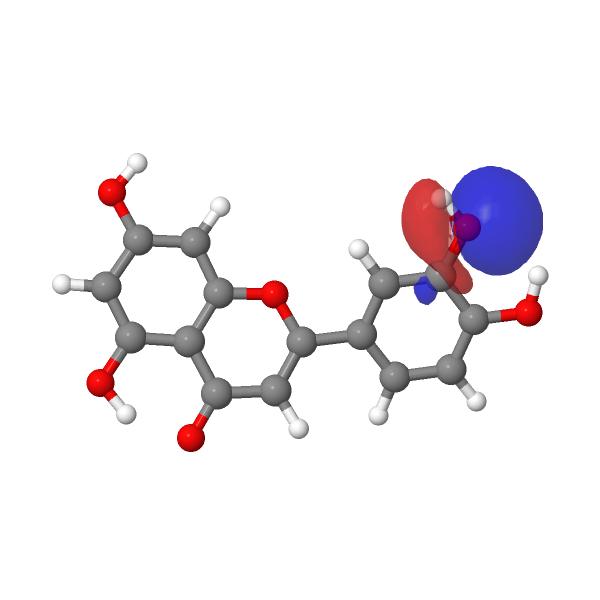 |
The calculated UV-Vis spectrum is shown below, showing the peak at ~300 NM responsible for the intense yellow colour (300-400 nm).
The strongest oscillator contribution to the transition is shown below.
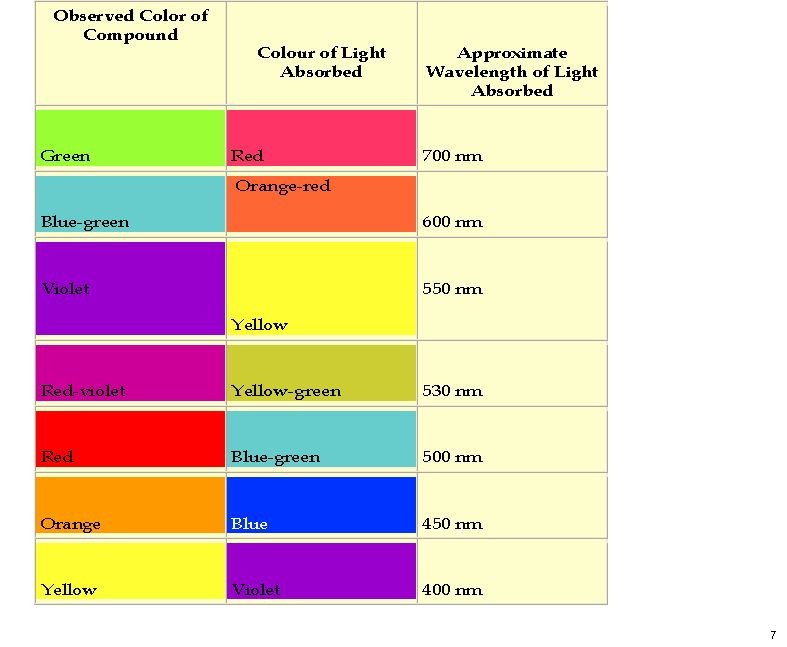
| LUMO au | HOMO |
|---|---|
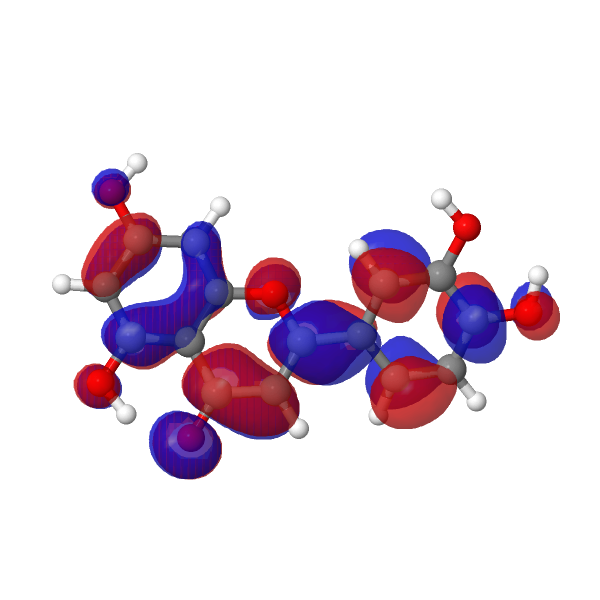 |
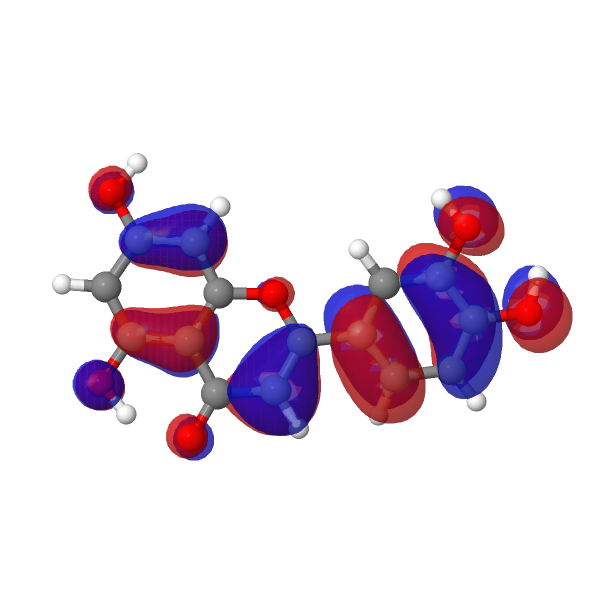 |
So here I have cast a little more light on this relatively unknown natural yellow dye, that was used for many centuries to colour woollen materials.
References
- C. Chavanne, A. Verney, C. Paquier-Berthelot, M. Bostal, P. Buléon, and P. Walter, "Bayeux Tapestry: First use of early synthetic dyes for the restoration of a masterpiece", Dyes and Pigments, vol. 208, pp. 110798, 2023. https://doi.org/10.1016/j.dyepig.2022.110798
- A.G. Perkin, "XLIX.—Luteolin. Part II", J. Chem. Soc., Trans., vol. 69, pp. 799-803, 1896. https://doi.org/10.1039/ct8966900799