The term bispericyclic reaction was famously coined by Caramella et al in 2002[1] to describe the unusual features of the apparently innocuous dimerisation of cyclopentadiene. It shows features of two paths for different pericyclic reactions, comprising a 2+4 cycloaddition in the early stages, but evolving into a (degenerate) pair of [3,3] sigmatropic reactions in the latter stages. Houk (who also uses the term ambimodal) has in recent years extended the number of examples of such pericyclic sequences to trispericyclic[2] (see here) and even an ambimodel tetrapericyclic reaction, as reported at the recent WATOC event. Here I show an example of a new type of bispericyclic reaction, comprising a 2+4 cycloaddition combined with a electrocyclic ring opening.
The reaction starts with cyclopropanone. This species is reported to undergo cycloaddition reactions[3] with e.g. furan; below is shown the scheme with butadiene.
Calculations at the ωB97XD/Def2-TZVPP/SCRF=DCM were undertaken (FAIR data DOI 10.14469/hpc/11246). Because it has been suggested that the “intermediate” ylid might have some biradical character, this was modelled using the option of a spin-unrestricted (UHF) method and a starting guess for the density matrix using the Gaussian keyword guess(mix), which uses a linear combination of the HOMO and LUMO to destroy α-β and spatial symmetries. At the located transition state, the spin expectation operator <S**2>= 0.4003 and it reaches a maximum value of <S**2>= 0.7002, so this system does indeed have some biradical character (the value would be 0.000 for a closed shell system, and 1.000 for a pure biradical). The IRC is shown below.
<S**2> is non-zero only between the range -2.5 to +4.5, the rest of the path has the value <S**2> =0.0. The central region of the path (IRC ~0 to +4.5) shows very small gradients, and can be characterised as the region corresponding to the ylid above acting as a “hidden intermediate”.
The central region also coincides with an increase in the dipole moment at the start of the reaction and a decrease at the end indicating that the system also has a fair amount of zwitterionic character, as implied in the ylid structure shown above. But the dip in dipole moment at around IRC = 2 corresponds to biradical character partially replacing the charge-separated ionic mode.
The IRC animation shown below shows that in the early stages of the reaction, electrocyclic ring opening (with disrotation) occurs, but no explicit ylid intermediate is formed. Instead the reaction continues without pause to a cycloaddition reaction for completion.

One interesting question is what impact does the partial biradical character have on this outcome? Shown below are the IRC responses to a potential for which the wavefunction has <S**2> =0.0 across the entire region.
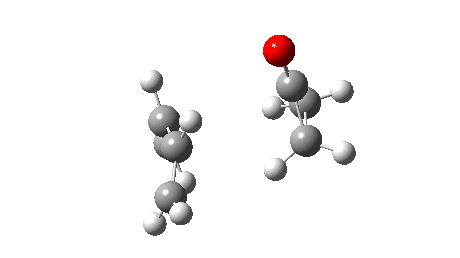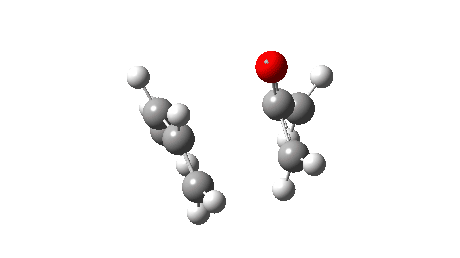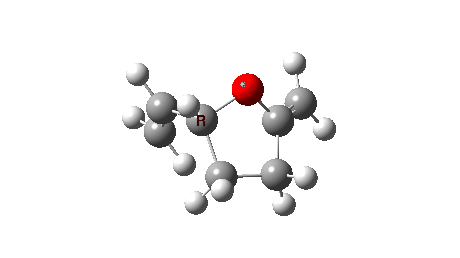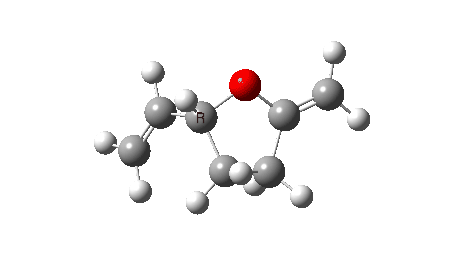
The reaction reveals an entirely different, but still bis-pericyclic reaction involving the same electrocyclic ring opening to the ylid shown above, but then reaction as a 2+2 cycloaddition reaction via the oxygen rather than the carbon of the ylid. The dipole moment no longer shows the biradical-induced dip in the middle, but rises to a full maximum instead.
So here we see a sequential bispericyclic reaction in which the product of an initial electrocyclic pericyclic reaction is so reactive that it immediately reacts with a diene to form the final product, in an overall concerted but asynchronous reaction. Modelling this seems to be very method dependent, and the apparently correct product is only obtained if an open-shell biradical wavefunction is used. Are there more examples?
This post has DOI: 10.14469/hpc/11247
References
- P. Caramella, P. Quadrelli, and L. Toma, "An Unexpected Bispericyclic Transition Structure Leading to 4+2 and 2+4 Cycloadducts in the <i>E</i><i>ndo</i> Dimerization of Cyclopentadiene", Journal of the American Chemical Society, vol. 124, pp. 1130-1131, 2002. https://doi.org/10.1021/ja016622h
- X. Xue, C.S. Jamieson, M. Garcia-Borràs, X. Dong, Z. Yang, and K.N. Houk, "Ambimodal Trispericyclic Transition State and Dynamic Control of Periselectivity", Journal of the American Chemical Society, vol. 141, pp. 1217-1221, 2019. https://doi.org/10.1021/jacs.8b12674
- H.H. Wasserman, D.R. Berdahl, and T. Lu, "The Chemistry of Cyclopropanones", Cyclopropyl Group Volume 1 and Volume 2 (1987), pp. 1455-1532, . https://doi.org/10.1002/0470023449.ch23