I noted in an earlier post the hypothesized example of (CO)3Fe⩸C[1] as exhibiting a carbon to iron quadruple bond and which might have precedent in known five-coordinate metal complexes where one of the ligands is a “carbide” or C ligand. I had previously mooted that the Fe⩸C combination might be replaceable by an isoelectronic Mn⩸N pair which could contain a quadruple bond to the nitrogen. An isoelectronic alternative to FeC could also be FeN+. Here I explore the possibility of realistic candidates for such bonded nitrogen.
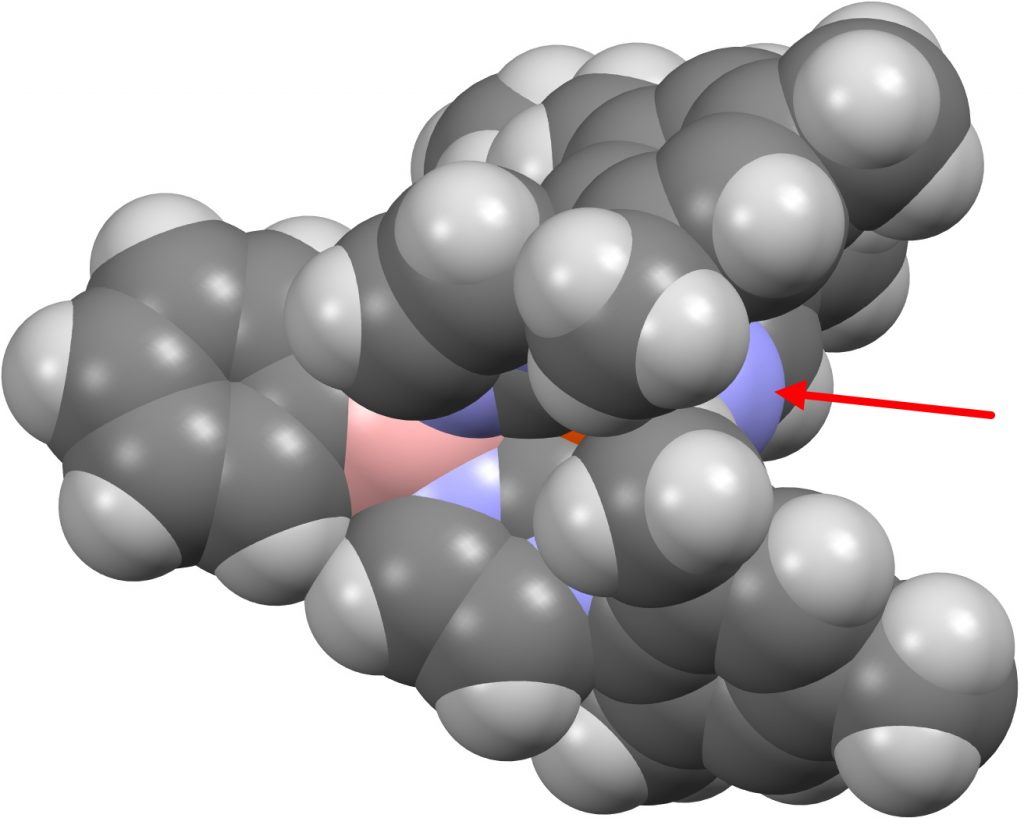
So I follow the strategy set in the previous post of conducting a crystal structure search of molecules containing the sub-structure L3-MN or L4-MN. Of the 85 hits for the former (FAIR DOI 10.14469/hpc/8196), I focus on those where N has only one bonded atom (to the metal M) and the ligand L is non-anionic connecting to the metal via e.g. carbon or phosphorus. This reduces to 11 hits, which in fact contain something similar to the Arduengo “carbene” ligand L shown below, this being known as a phosphine replacement. Here I look at one of these molecules, the internal ion-pair where the positive charge on the N is balanced by a four-coordinate negative boron, as in HAQLET.[2] (Data DOI: 10.5517/ccdc.csd.cc1p0mp0).
As with (CO)3Fe⩸C, L3Fe⩸N+ has a filled 18-electron metal valence shell. A ωB97XD/Def2-SVPD calculation on a simplified model (with aryl groups replaced by H) reveals the following NBO localised orbitals.
| M-N, r = 1.475Å. | |
|---|---|
| NBO 72, Occupied, Non-bonding d-orbital | NBO 71, Occupied, Non-bonding d-orbital |
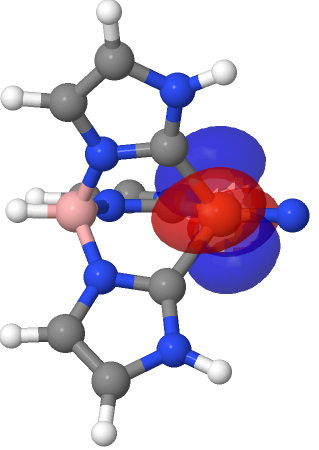 |
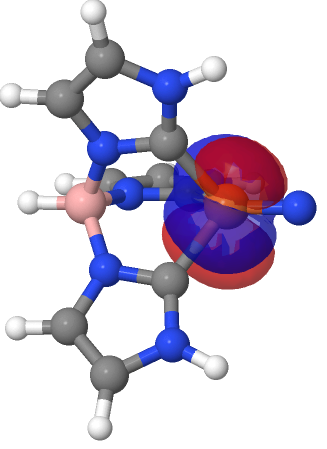 |
| NBO 67 π bond | NBO 66 π bond |
 |
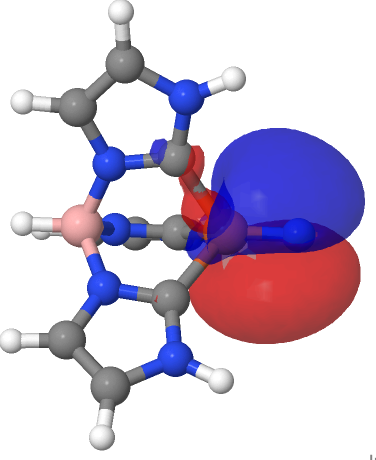 |
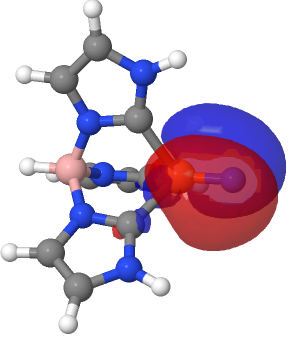
| NBO 59 σ bond | NBO 27 σ bond |
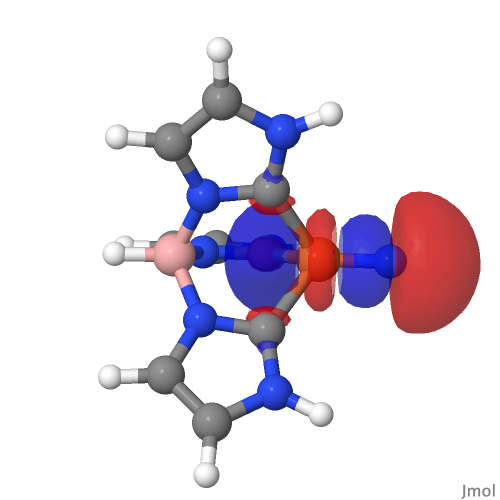 |
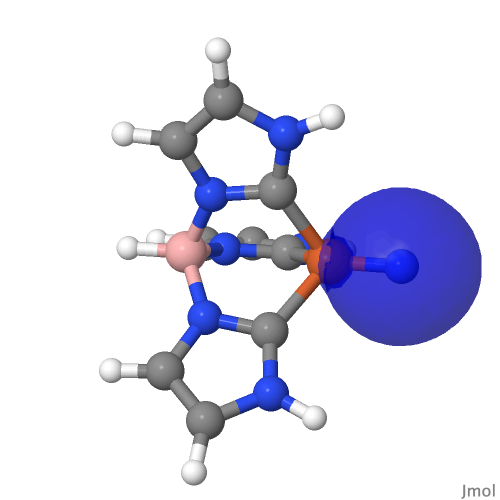 |
There are two σ-bonds and two π-bonds between the Fe and the N. The molecule is presumably inhibited from reaction such as e.g. dimerising, because the iron-bonded nitrogen atom sits in a well created by the mesityl groups, thus sterically preventing any N…N approach close enough and at the appropriate angle to unite the two units. The free energy of dimerisation of the unhindered model used above is -49.7 kcal/mol.
I remind that the NBO method being used to ascertain the nature of the bonding here is a binary method, giving localised NBO orbitals with ~2e occupancies that contain an integer number of bonding orbitals between any pair of atoms. In this case, these can point to either a triple or a quadruple M…N bond for such systems and do not allow for a continuum approach where the weight of each localised bond might not be close to an integer. The purpose here is to flag this system for further analysis rather than as a definitive declaration of its quadruple-bonded nature.
References
- A.J. Kalita, S.S. Rohman, C. Kashyap, S.S. Ullah, and A.K. Guha, "Transition metal carbon quadruple bond: viability through single electron transmutation", Physical Chemistry Chemical Physics, vol. 22, pp. 24178-24180, 2020. https://doi.org/10.1039/d0cp03436c
- L. Bucinsky, M. Breza, W. Lee, A.K. Hickey, D.A. Dickie, I. Nieto, J.A. DeGayner, T.D. Harris, K. Meyer, J. Krzystek, A. Ozarowski, J. Nehrkorn, A. Schnegg, K. Holldack, R.H. Herber, J. Telser, and J.M. Smith, "Spectroscopic and Computational Studies of Spin States of Iron(IV) Nitrido and Imido Complexes", Inorganic Chemistry, vol. 56, pp. 4751-4768, 2017. https://doi.org/10.1021/acs.inorgchem.7b00512