Way back in 2010, I was writing about an experience I had just had during an organic chemistry tutorial, which morphed into speculation as to whether a carbon atom might sustain a quadruple bond to nitrogen. A decade on, and possibly approaching 100 articles by many authors on the topic, quadruple bonds to carbon continue to fascinate. Now an article as appeared[1] repeating this speculation for a carbon to iron quadruple bond,‡ in the very simple species C⩸Fe(CO)3 (see also a Rh-B equivalent[2]). This is particularly exciting because of the very real prospect of synthesising this species and perchance getting a crystal structure (something not possible with most of the other quadruply bonded carbon systems studied to date).
They authors report[1] that this sytem is well described by a single-configurational wavefunction and hence that a M062X/Def2-TZVPP calculation is a good description of the bonding. In the article you will find the valence molecular orbitals shown, which by their nature show a lot of delocalisation. Because the more localised NBOs are not shown in the article, I illustrate them here, as 3D rotatable models. The DOI for the calculation can be found at 10.14469/hpc/7537.
| CFe(CO)3 | |
|---|---|
| NBO 37 π | NBO 36 π |
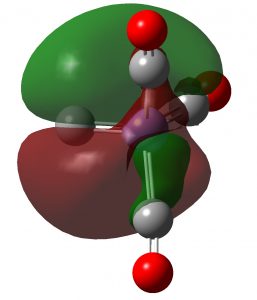 Click to view 3D model of NBO 37 |
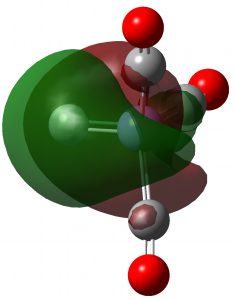 Click to view 3D model of NBO 36 |
| NBO 33 σ | NBO 23 σ |
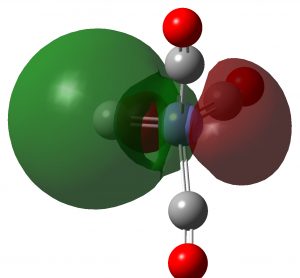 Click to view 3D model of NBO 33 |
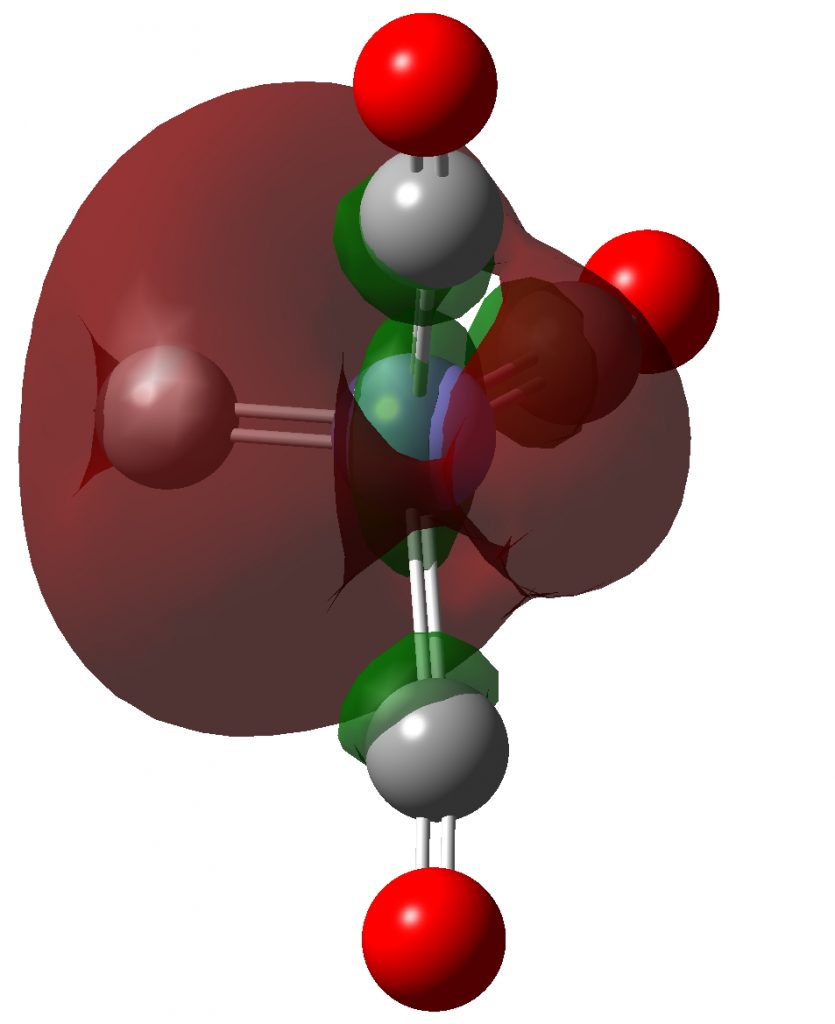 Click to view 3D model of NBO 23 |
These NBOs show very clearly that the two higher energy orbitals are orthogonal π-bonds to carbon from Fe and the two lower energy orbitals are both σ-bonds to carbon from Fe. Exactly the same picture appears for C2, which has been often mentioned on this blog.
All that remains is that some inspired synthetic chemist sets out to make C⩸Fe(CO)3 and to report back on its properties. I fancy the last has not yet been heard about quadruple bonds to carbon!
Postscript: Here are the analogous orbitals for the species N⩸Mn(CO)3 to illustrate their evolution across an isoelectronic series.
| NMn(CO)3 | |
|---|---|
| NBO 35 | NBO 34 |
 Click to view 3D model of NBO 35 |
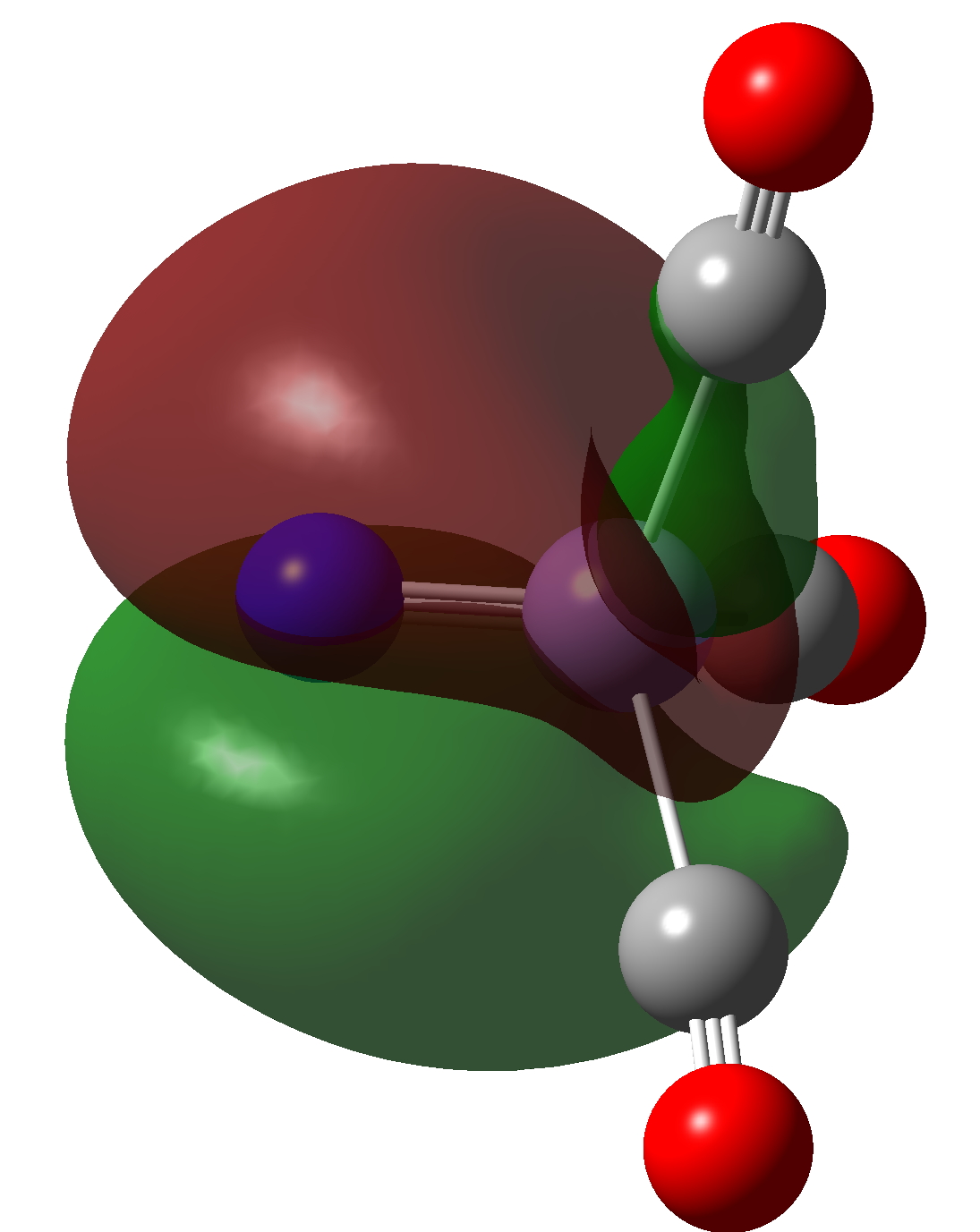 Click to view 3D model of NBO 34 |
| NBO 33 | NBO 20 |
 Click to view 3D model of NBO 33 |
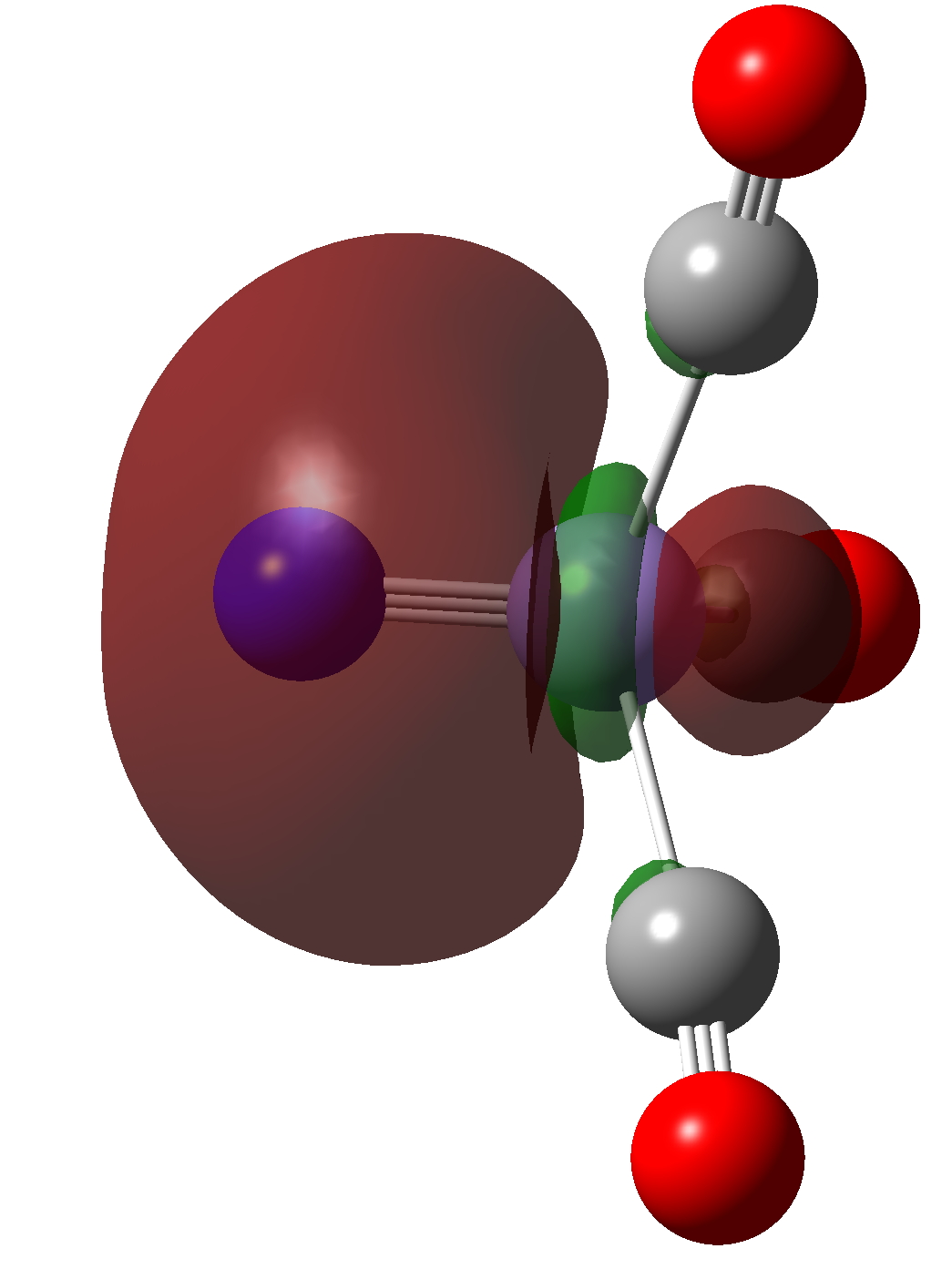 Click to view 3D model of NBO 20 |
NBO 33 (the higher in energy of the two σ-type bonds) has an interesting structure. Here it is at a slightly lower threshold:
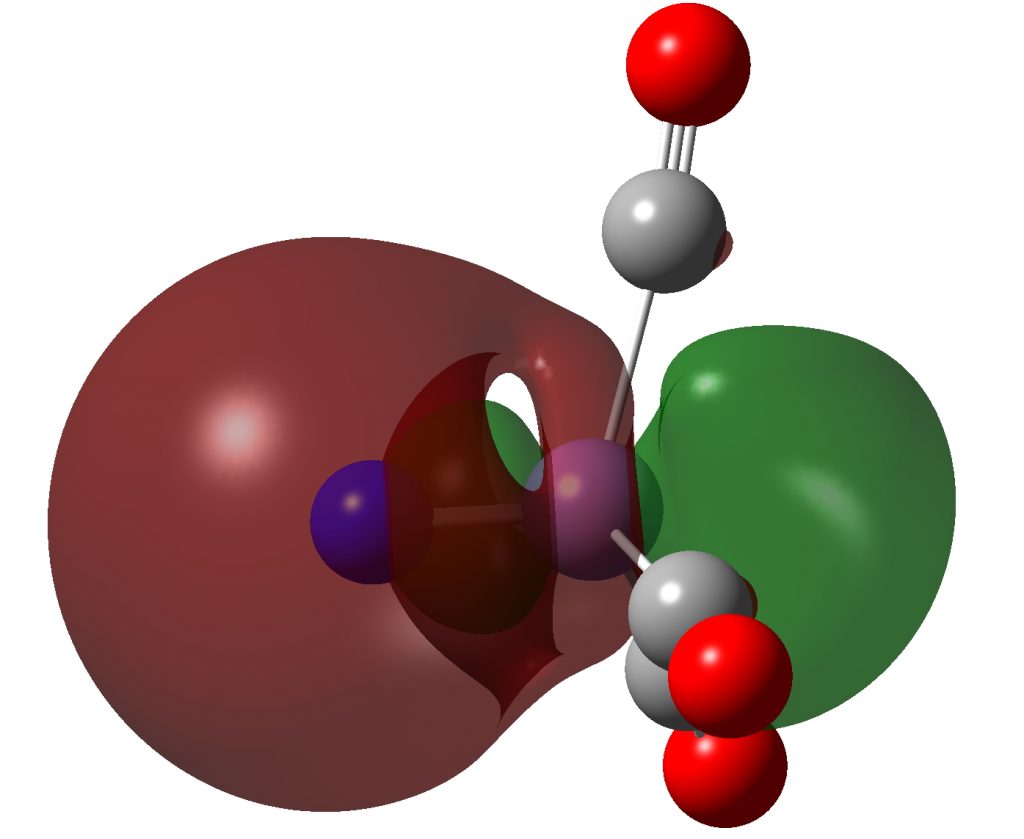
Click to view 3D model of NBO 33 for NMn(CO)3 at threshold 0.015au
It has an inner compact σ-bond running along the axis of the bond and an outer “wrap” of different phase. A two layer σ-bond if you like!
‡This was one of the shortlisted candidates for Molecule of the Year, 2020. See also[3]
References
- A.J. Kalita, S.S. Rohman, C. Kashyap, S.S. Ullah, and A.K. Guha, "Transition metal carbon quadruple bond: viability through single electron transmutation", Physical Chemistry Chemical Physics, vol. 22, pp. 24178-24180, 2020. https://doi.org/10.1039/d0cp03436c
- L.F. Cheung, T. Chen, G.S. Kocheril, W. Chen, J. Czekner, and L. Wang, "Observation of Four-Fold Boron–Metal Bonds in RhB(BO<sup>–</sup>) and RhB", The Journal of Physical Chemistry Letters, vol. 11, pp. 659-663, 2020. https://doi.org/10.1021/acs.jpclett.9b03484
- A.J. Kalita, S.S. Rohman, C. Kashyap, S.S. Ullah, I. Baruah, L.J. Mazumder, P.P. Sahu, and A.K. Guha, "Is a transition metal–silicon quadruple bond viable?", Physical Chemistry Chemical Physics, vol. 23, pp. 9660-9662, 2021. https://doi.org/10.1039/d1cp00598g
If the Boron-Cobalt analogue also has a quadruple bond it would be a pretty unusual molecule. Boron in the formally -5 oxidation state, and singly coordinated.