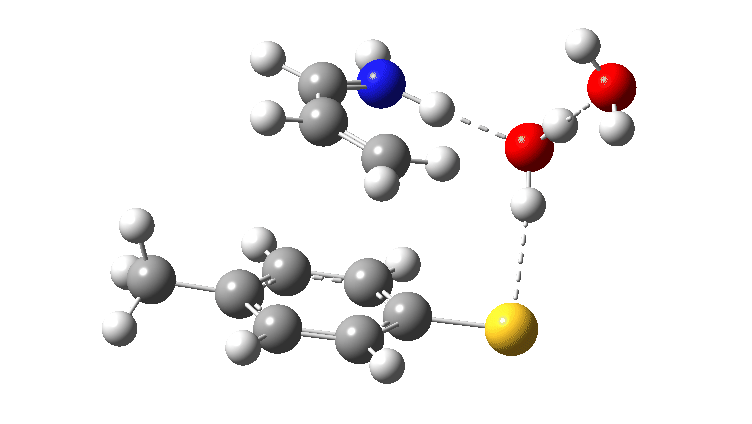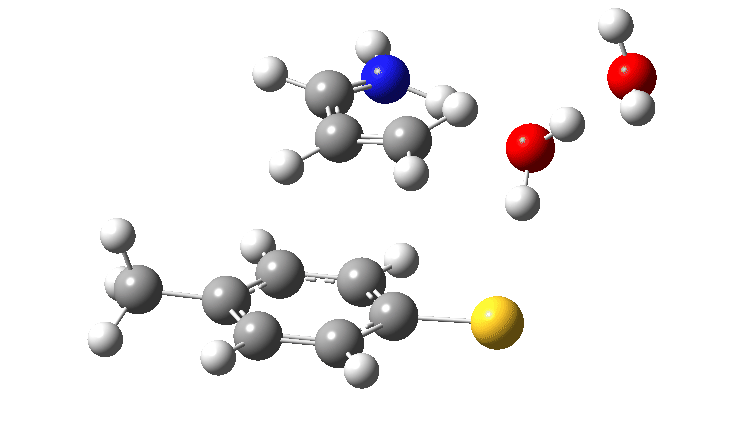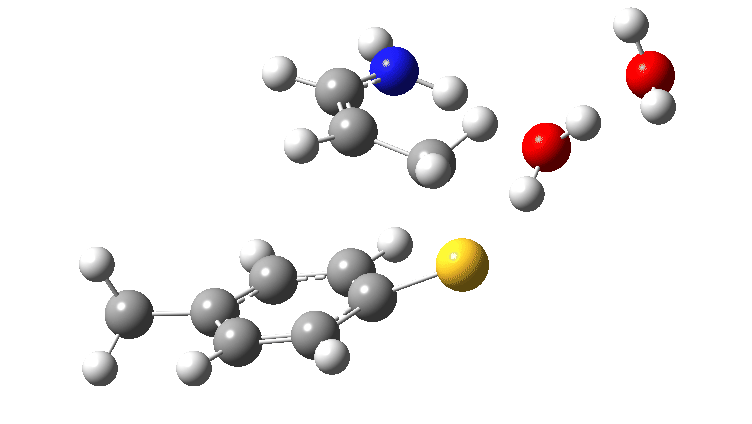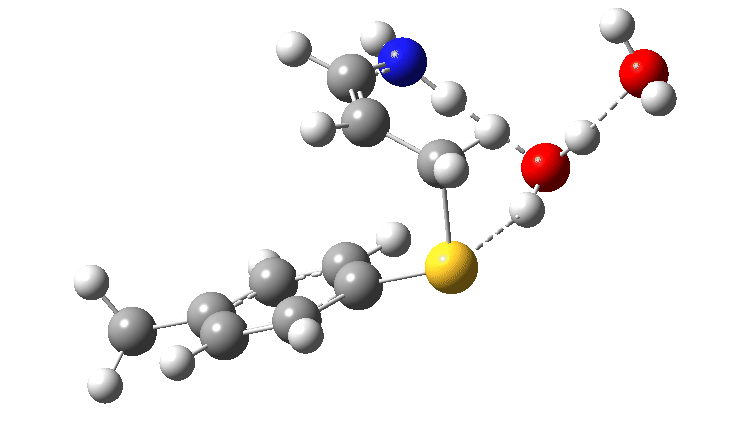
Earlier, I explored the choreography or “timing”, of what might be described as the curly arrows for a typical taught reaction mechanism, the 1,4-addition of a nucleophile to an unsaturated carbonyl compound (scheme 1). I am now going to explore the consequences of changing one of the actors by adding the nucleophile to an unsaturated imine rather than carbonyl compound (scheme 2).
For the reaction shown in Scheme 1, the maximum energy point along the reaction path involves the formation of an S-C bond (arrow 2 in scheme 1) rather than transfer of a proton. Scheme 2 has a new actor in which NH replaces O and which is a better base (i.e. has a greater affinity for a proton). The mechanism again starts with arrows 1 and 2 launching proceedings. If you watch the animation below very carefully, you will notice that arrows 3 and 4 lag behind them. This means that you have to have the blue arrows 1 and 4 as distinctly separate arrows. An alternative depiction (and in truth very probably the depiction you would find in pretty much all text books and lecture notes) would be to combine arrows 1 and 4 into the single red arrow 8. If you do this however, you loose this subtle nuance to the mechanism.
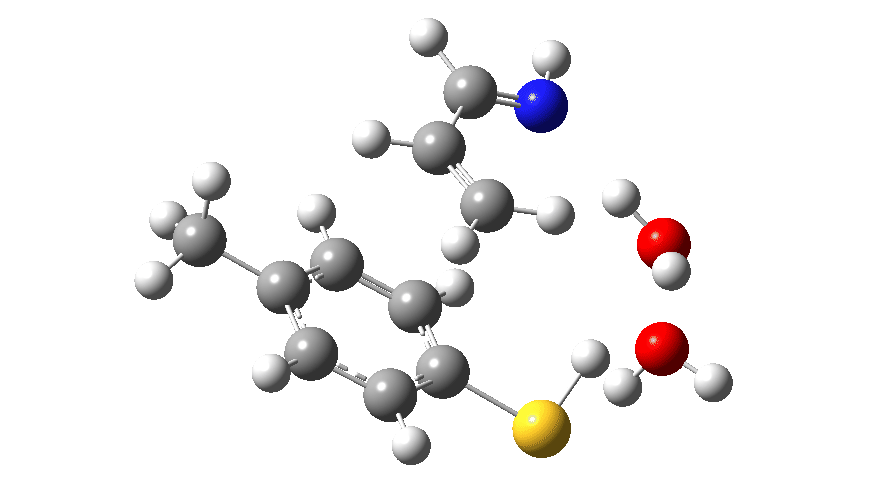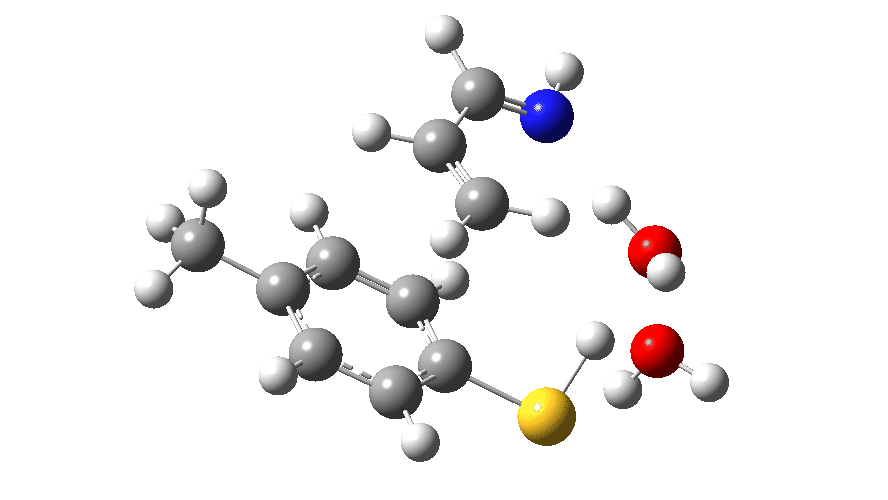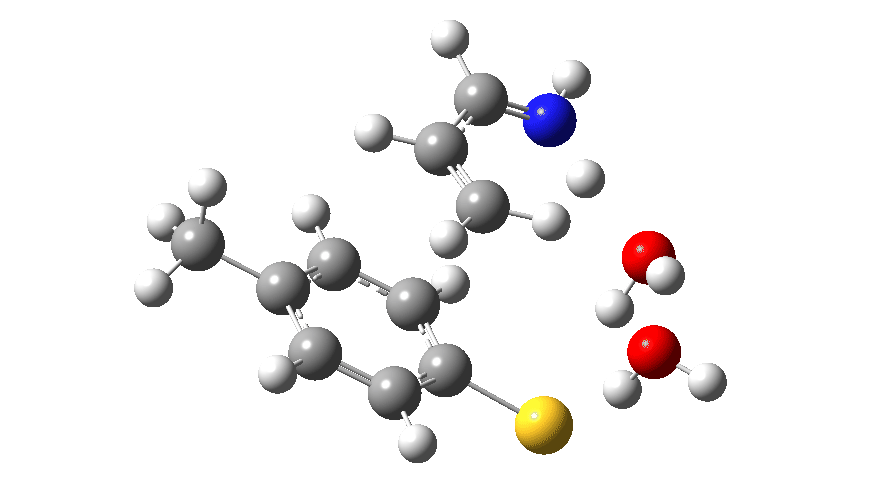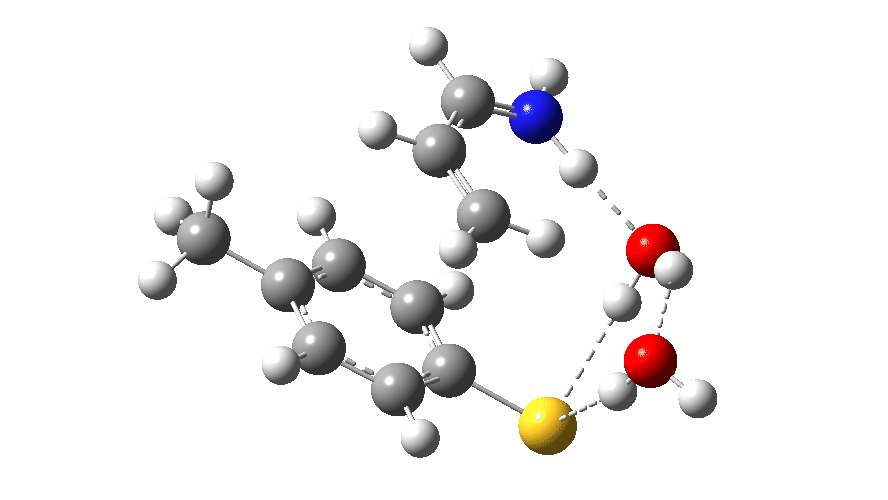
Animated Reaction coordinate for TS1 (scheme 2) Click to load 3D model
The product of this first step is a zwitterionic (internal ion-pair) compound. This then goes on to form the S-C bond (arrows 5–7) via TS2, with the energy of this second transition state being lower than than TS1.
The free energy barrier for this second step is low (ΔG† 0.6 kcal/mol) because it is an ion-pair reacting to form a neutral molecule, always a facile process. Because the slowest step in this reaction (TS1) involves a proton transfer, this should now show a primary deuterium kinetic isotope effect. Indeed this is calculated to have the value 4.0 at 298K. There is a prediction for an experiment to undertake!
| Species (FAIR Data: dt5d) |
Relative energy kcal/mol |
|---|---|
| Reactant | 0.0 |
| TS1 | 7.7 |
| Zwitterion | 2.2 |
| TS2 | 2.8 |
| Product | -12.5 |
To sumarise
- Changing a C=O group to a C=NH group changes the nature of the mechanism from concerted asynchrous to stepwise.
- As a result of this change, the highest energy step now involves asynchronous proton transfers rather than S-C bond formation.
- The curly arrows can be used to reflect these steps, with two (blue) arrows being preferred to a single (red) one.
So by expanding the conventional number of curly arrows used to include extra ones capturing asynchronicity in the reaction, one can indeed add further information to the curly arrow formalism.
This post has DOI: dt6v