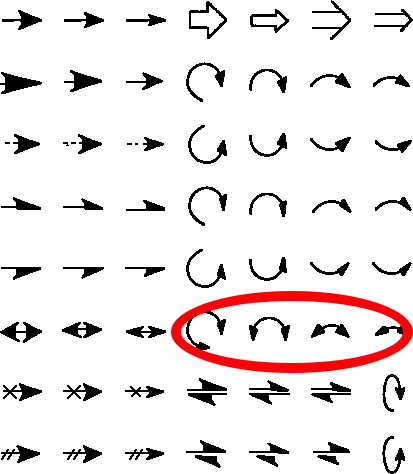
The schematic representation of a chemical reaction mechanism is often drawn using a palette of arrows connecting or annotating the various molecular structures involved. These can be selected from a chemical arrows palette, taken for this purpose from the commonly used structure drawing program Chemdraw. Explanations of how to apply the individual arrows are not always easy to find however! Circled in red are the ones to be discussed here, although most carry fascinating and often subtle meanings!‡
Archive for the ‘Curly arrows’ Category
The “double-headed” curly arrow as used in mechanistic representations.
Tuesday, August 29th, 2023Pre-mechanism for the Swern Oxidation: formation of chlorodimethylsulfonium chloride.
Friday, August 25th, 2023The Swern oxidation[1] is a class of “activated” dimethyl sulfoxide (DMSO) reaction in which the active species is a chlorodimethylsulfonium chloride salt. The mechanism of this transformation as shown in e.g. Wikipedia is illustrated below.‡ However, an interesting and important aspect of chemistry is not apparent in this schematic mechanism and to rectify this, a full computed mechanism is laid out below, for which the FAIR data has a DOI: 10.14469/hpc/13151
(more…)
References
- K. Omura, and D. Swern, "Oxidation of alcohols by “activated” dimethyl sulfoxide. a preparative, steric and mechanistic study", Tetrahedron, vol. 34, pp. 1651-1660, 1978. https://doi.org/10.1016/0040-4020(78)80197-5
Dimerisation of cyclopropenylidene: what are the correct “curly arrows” for this process?
Wednesday, July 21st, 2021In another post, a discussion arose about whether it might be possible to trap cyclopropenylidene to form a small molecule with a large dipole moment. Doing so assumes that cyclopropenylidene has a sufficiently long lifetime to so react, before it does so with itself to e.g. dimerise. That dimerisation has an energy profile shown below, with a free energy of activation of 14.4 kcal/mol, so a facile reaction that will indeed compete with reaction with Ph-I+-CC–.
Trimerous pericyclic reactions.
Thursday, October 8th, 2020I occasionally spot an old blog that emerges, if only briefly, as “trending”. In this instance, only the second blog I ever wrote here, way back in 2009 as a follow up to this article.[1] With something of that age, its always worth revisiting to see if any aspect needs updating or expanding, given the uptick in interest. It related to the observation that there can be more than one way of expressing the “curly arrows” for some pericyclic reactions. These alternatives may each represent different types of such reactions, hence leading to a conundrum for students of how to label the mechanism. I had noted in that blog that I intended to revisit the topic and so a mere eleven years later here it is!
References
- H.S. Rzepa, "The Aromaticity of Pericyclic Reaction Transition States", Journal of Chemical Education, vol. 84, pp. 1535, 2007. https://doi.org/10.1021/ed084p1535
Curly arrows in the 21st Century. Proton-coupled electron transfers.
Wednesday, June 10th, 2020One of the most fascinating and important articles dealing with curly arrows I have seen is that by Klein and Knizia on the topic of C-H bond activations using an iron catalyst.[1] These are so-called high spin systems with unpaired electrons and the mechanism of C-H activation involves both double headed (two electron) and fish-hook (single electron) movement. Here I focus on a specific type of reaction, the concerted proton-coupled-electron transfer or cPCET, as illustrated below. These sorts of reactions happen also to be of considerable biological importance, including e.g. the mechanism of photosynthesis and many other important transformations.
References
- J.E.M.N. Klein, and G. Knizia, "cPCET versus HAT: A Direct Theoretical Method for Distinguishing X–H Bond‐Activation Mechanisms", Angewandte Chemie International Edition, vol. 57, pp. 11913-11917, 2018. https://doi.org/10.1002/anie.201805511
The first ever curly arrows. Revisited with some crystal structure mining.
Wednesday, May 27th, 2020With the current global lockdown, and students along with everyone else staying at home, I have noticed some old posts of mine are getting more attention than normal. One of these is an analysis I did in 2012 of Robinson’s original curly arrow illustration.[1] That and the fact that I am about to give a lecture on what I call my autobiographical journey discovering them, to our own students here (remotely of course), has prompted me to revisit my original discussion.
References
- "Forthcoming events", Journal of the Society of Chemical Industry, vol. 43, pp. 1295-1298, 1924. https://doi.org/10.1002/jctb.5000435208
Choreographing a chemical ballet: what happens if you change one of the actors?
Friday, May 8th, 2020Earlier, I explored the choreography or “timing”, of what might be described as the curly arrows for a typical taught reaction mechanism, the 1,4-addition of a nucleophile to an unsaturated carbonyl compound (scheme 1). I am now going to explore the consequences of changing one of the actors by adding the nucleophile to an unsaturated imine rather than carbonyl compound (scheme 2).
Choreographing a chemical ballet: a story of the mechanism of 1,4-Michael addition.
Monday, April 13th, 2020A reaction can be thought of as molecular dancers performing moves. A choreographer is needed to organise the performance into the ballet that is a reaction mechanism. Here I explore another facet of the Michael addition of a nucleophile to a conjugated carbonyl compound. The performers this time are p-toluene thiol playing the role of nucleophile, adding to but-2-enal (green) acting as the electrophile and with either water or ammonia serving the role of a catalytic base to help things along.†
Substituent effects on the mechanism of Michael 1,4-Nucleophilic addition.
Sunday, March 29th, 2020In the previous post, I looked at the mechanism for 1,4-nucleophilic addition to an activated alkene (the Michael reaction). The model nucleophile was malonaldehyde after deprotonation and the model electrophile was acrolein (prop-2-enal), with the rate determining transition state being carbon-carbon bond formation between the two, accompanied by proton transfer to the oxygen of the acrolein.
The Graham reaction: Deciding upon a reasonable mechanism and curly arrow representation.
Monday, February 18th, 2019Students learning organic chemistry are often asked in examinations and tutorials to devise the mechanisms (as represented by curly arrows) for the core corpus of important reactions, with the purpose of learning skills that allow them to go on to improvise mechanisms for new reactions. A common question asked by students is how should such mechanisms be presented in an exam in order to gain full credit? Alternatively, is there a single correct mechanism for any given reaction? To which the lecturer or tutor will often respond that any reasonable mechanism will receive such credit. The implication is that a mechanism is “reasonable” if it “follows the rules”. The rules are rarely declared fully, but seem to be part of the absorbed but often mysterious skill acquired in learning the subject. These rules also include those governing how the curly arrows should be drawn.† Here I explore this topic using the Graham reaction.[1]‡
References
- W.H. Graham, "The Halogenation of Amidines. I. Synthesis of 3-Halo- and Other Negatively Substituted Diazirines<sup>1</sup>", Journal of the American Chemical Society, vol. 87, pp. 4396-4397, 1965. https://doi.org/10.1021/ja00947a040