Archive for the ‘reaction mechanism’ Category
Tuesday, December 16th, 2025
An investigation of the kinetics of the reaction between titanocene pentasulfide and sulfenyl chloride[1] leading to the formation of the S7 allotrope of sulfur was accompanied by supporting DFT calculations which led to the conclusion that of five possible mechanisms for the reaction, the most probable corresponded to a variant of the concerted σ-bond metathesis (Scheme 1, Mechanism IV, R = Cl). Here we take a closer look at the DFT predictions from the point of view of the impact of continuum solvation on the calculated mechanism.
(more…)
References
- P.H. Helou de Oliveira, P.J. Boaler, G. Hua, N.M. West, R.T. Hembre, J.M. Penney, M.H. Al-Afyouni, J.D. Woollins, A. García-Domínguez, and G.C. Lloyd-Jones, "Kinetics of sulfur-transfer from titanocene (poly)sulfides to sulfenyl chlorides: rapid metal-assisted concerted substitution", Chemical Science, vol. 15, pp. 11875-11883, 2024. https://doi.org/10.1039/d4sc02737j
Posted in reaction mechanism | No Comments »
Friday, November 21st, 2025
Substituting a deuterium isotope (2H) for a normal protium hydrogen isotope can slow the rate of a chemical reaction if this atom is involved in the reaction mode. The magnitude of the effect, referred to as a kinetic isotope effect or KIE is normally 2-7, but higher values of 20 or even more♥ are sometimes observed due to a phenomenon known as proton tunnelling. So a recent report[1] of a 1H/2H of ~2440 for the following palladium catalysed reaction caught my eye:
(more…)
References
- Q. Wu, P. Liu, X. Zhang, C. Fan, Z. Chen, R. Qin, Y.Q. Gao, Y. Zhao, and N. Zheng, "Catalytic Hydrogenation Dominated by Concerted Hydrogen Tunneling at Room Temperature", ACS Central Science, vol. 11, pp. 2180-2187, 2025. https://doi.org/10.1021/acscentsci.5c00943
Posted in reaction mechanism | No Comments »
Tuesday, October 21st, 2025
Part one of this topic was posted more than ten years ago.[1] I clearly forgot about it, so belatedly, here is part 2 – dealing with the stereochemistry of the reduction of tert-butyl-cyclohexanone by borohydride in water. The known stereochemistry is nicely summarised in this article, along with an extensive history of possible explanations of the reasons for the stereochemical preference.[2] Put simply, the hydride nucleophile attacks the carbonyl from an axial rather than equation direction with a ratio of 10:1 (ΔΔG 1.37 kcal/mol). So does the model I previously proposed[1] support this and give any indication of why the stereochemistry is axial?
(more…)
References
- H. Rzepa, "Part 1: ethanal.", 2015. https://doi.org/10.59350/aqrgh-jw887
- R. Kobetić, V. Petrović-Peroković, V. Ključarić, B. Juršić, and D.E. Sunko, "Selective Reduction of Cyclohexanones with NaBH<sub>4</sub> in β-Cyclodextrin, PEG-400, and Micelles", Supramolecular Chemistry, vol. 20, pp. 379-385, 2008. https://doi.org/10.1080/10610270701268815
Posted in reaction mechanism | No Comments »
Tuesday, July 22nd, 2025
In the previous post[1] I followed up on an article published on the theme “Physical Organic Chemistry: Never Out of Style“.[2] Paul Rablen presented the case that the amount of o (ortho) product in electrophilic substitution of a phenyl ring bearing an EWG (electron withdrawing group) is often large enough to merit changing the long held rule-of-thumb for EWGs from being just meta directors into being ortho and meta-directors, with a preference for meta. I showed how Paul’s elegant insight could be complemented by an NBO7 analysis of the donor-acceptor interactions in the σ-complex formed by protonating the phenyl ring bearing the EWG. Both the o– and m– isomers showed similar NBO orbital patterns and associated E(2) donor/acceptor interaction energies and also matched the observation that the proportion of meta is modestly greater than ortho substitution (steric effects not modelled). These interactions were both very different from those calculated for the para isomer.
(more…)
References
- H. Rzepa, ""Typical Electron-Withdrawing Groups Are o, m-Directors Rather than m-Directors in Electrophilic Aromatic Substitution"", 2025. https://doi.org/10.59350/rzepa.28993
- P.R. Rablen, "Typical Electron-Withdrawing Groups Are <i>ortho</i>, <i>meta</i>-Directors Rather than <i>meta</i>-Directors in Electrophilic Aromatic Substitution", The Journal of Organic Chemistry, vol. 90, pp. 6090-6093, 2025. https://doi.org/10.1021/acs.joc.5c00426
Posted in reaction mechanism | No Comments »
Thursday, July 17th, 2025
The title of this post comes from an article published in a special virtual issue on the theme “Physical Organic Chemistry: Never Out of Style“[1] There, Paul Rablen presents the case that the amount of o (ortho) product in electrophilic substitution of a phenyl ring bearing an EWG (electron withdrawing group) is often large enough to merit changing the long held rule-of-thumb for EWGs from being just meta directors into these substituents are best understood as ortho, meta-directors, with a preference for meta. I cannot help but add here a citation[2] to the earliest publication I can find showing tables of both o,p and m-directing groups and dating from 1887, so this rule is 138 years old (at least).
(more…)
References
- P.R. Rablen, "Typical Electron-Withdrawing Groups Are <i>ortho</i>, <i>meta</i>-Directors Rather than <i>meta</i>-Directors in Electrophilic Aromatic Substitution", The Journal of Organic Chemistry, vol. 90, pp. 6090-6093, 2025. https://doi.org/10.1021/acs.joc.5c00426
- H.E. Armstrong, "XXVIII.—An explanation of the laws which govern substitution in the case of benzenoid compounds", J. Chem. Soc., Trans., vol. 51, pp. 258-268, 1887. https://doi.org/10.1039/ct8875100258
Posted in reaction mechanism | No Comments »
Saturday, June 14th, 2025
I am in the process of revising my annual lecture to first year university students on the topic of “curly arrows”. I like to start my story in 1924, when Robert Robinson published the very first example[1] as an illustration of why nitrosobenzene undergoes electrophilic bromination in the para position of the benzene ring. I follow this up by showing how “data mining” can be used to see if this supports his assertion. I have used the very latest version of the CSD crystal structure database to update the version originally posted here in 2020.[2]
(more…)
References
- "Forthcoming events", Journal of the Society of Chemical Industry, vol. 43, pp. 1295-1298, 1924. https://doi.org/10.1002/jctb.5000435208
- H. Rzepa, "The first ever curly arrows. Revisited with some crystal structure mining.", 2020. https://doi.org/10.59350/c6thp-wqe69
Posted in reaction mechanism | No Comments »
Wednesday, May 21st, 2025
In this series of posts about the electronic effects in small sulfur rings[1] I have explored increasingly large induced geometric effects. Here is the largest so far, for the compound S7I1+[2]
(more…)
References
- H. Rzepa, "5-Imino-5λ<sup>4</sup>-heptathiepane 3-oxide. More exuberent anomeric effects.", 2025. https://doi.org/10.59350/rzepa.28615
- J. Passmore, G. Sutherland, P. Taylor, T.K. Whidden, and P.S. White, "Preparations and x-ray crystal structures of iodo-cyclo-heptasulfur hexafluoroantimonate(V) and hexafluoroarsenate(V), S7ISbF6 and S7IAsF6", Inorganic Chemistry, vol. 20, pp. 3839-3845, 1981. https://doi.org/10.1021/ic50225a048
Posted in Interesting chemistry, reaction mechanism | No Comments »
Monday, May 19th, 2025
The monosulfoxide of cyclo-heptasulfur was reported along with cycloheptasulfur itself in 1977,[1] along with the remarks that “The δ modification of S7 contains bonds of widely differing length: this has never been observed before in an unsubstituted molecule. and “the same effect having also been observed in other sulfur rings (S8O, S7I1+ and S7O).” Here I take a look at the last of these other molecules, the monosulfoxide of S7, as a follow up to the commentary on S7 itself.[2]
(more…)
References
- R. Steudel, R. Reinhardt, and T. Sandow, "Bond Interaction in Sulfur Rings: Crystal and Molecular Structure of <i>cyclo</i>‐Heptasulfur Oxide, S<sub>7</sub>O", Angewandte Chemie International Edition in English, vol. 16, pp. 716-716, 1977. https://doi.org/10.1002/anie.197707161
- H. Rzepa, "Cyclo-Heptasulfur, S<sub>7</sub> – a classic anomeric effect discovered during a pub lunch!", 2025. https://doi.org/10.59350/rzepa.28407
Posted in Interesting chemistry, reaction mechanism | No Comments »
Tuesday, August 29th, 2023
The schematic representation of a chemical reaction mechanism is often drawn using a palette of arrows connecting or annotating the various molecular structures involved. These can be selected from a chemical arrows palette, taken for this purpose from the commonly used structure drawing program Chemdraw. Explanations of how to apply the individual arrows are not always easy to find however! Circled in red are the ones to be discussed here, although most carry fascinating and often subtle meanings!‡
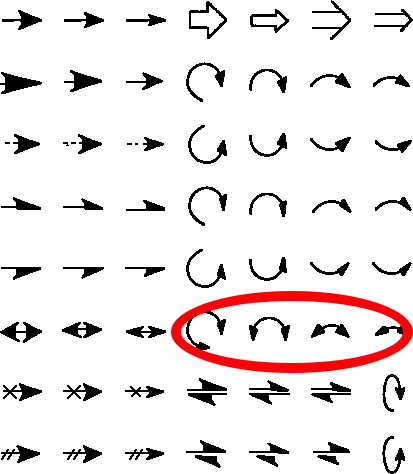
(more…)
Tags:Curly arrows
Posted in Curly arrows, reaction mechanism | No Comments »
Friday, August 25th, 2023
The Swern oxidation[1] is a class of “activated” dimethyl sulfoxide (DMSO) reaction in which the active species is a chlorodimethylsulfonium chloride salt. The mechanism of this transformation as shown in e.g. Wikipedia is illustrated below.‡ However, an interesting and important aspect of chemistry is not apparent in this schematic mechanism and to rectify this, a full computed mechanism is laid out below, for which the FAIR data has a DOI: 10.14469/hpc/13151
 (more…)
(more…)
References
- K. Omura, and D. Swern, "Oxidation of alcohols by “activated” dimethyl sulfoxide. a preparative, steric and mechanistic study", Tetrahedron, vol. 34, pp. 1651-1660, 1978. https://doi.org/10.1016/0040-4020(78)80197-5
Posted in Curly arrows, reaction mechanism | 3 Comments »
