Conformational polymorphism occurs when a compound crystallises in two polymorphs differing only in the relative orientations of flexible groups (e.g. Ritonavir).[1] At the Beilstein conference, Ian Bruno mentioned another type; tautomeric polymorphism, where a compound can crystallise in two forms differing in the position of acidic protons. Here I explore three such examples.
The term occurs in the title of this article,[2] for a compound known as Omeprazole.
When the bottom structure (the 6-methoxy) is used to search the CSD, two separate series are found. The first of these is UDAVIF (DOI: 10.5517/ccp82qq, 6-Methoxy-2-((4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl)-1H-benzimidazole). There is no information regarding the absolute configuration of the chiral S-centre. Although the downloaded coordinates show it as R it is probably a racemic mixture. A note added to the structure declares disorder: “Omeprazole exists as solid solutions of the two tautomers. The structure is mixed 5-methoxy/6-methoxy with occupancies 0.078:0.922“, which indicates 7.8% is present as in the upper structure above.
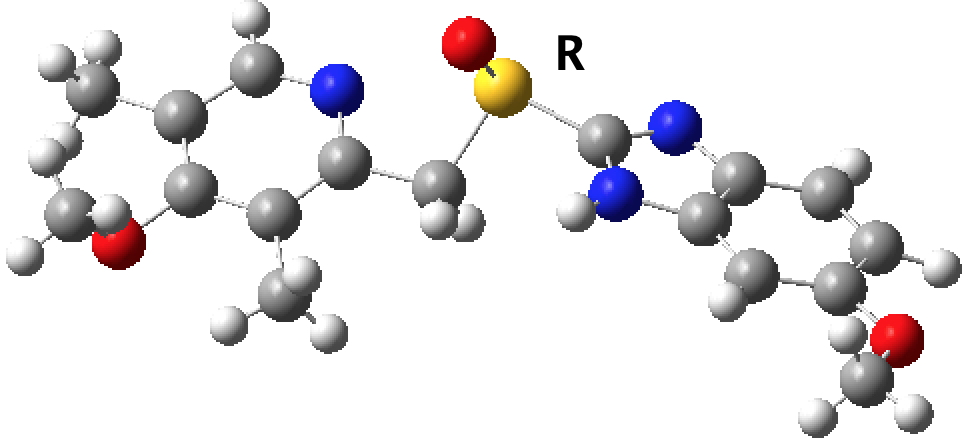
The second hit is VAYXOI (DOI: 10.5517/ccp82pp, rac-6-Methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-1H-benzimidazole) which now contains no disorder; the contaminating 5-methoxy tautomer is no longer present. Perhaps not quite a true tautomeric polymorph, since the 5-methoxy tautomer is never observed in pure form.
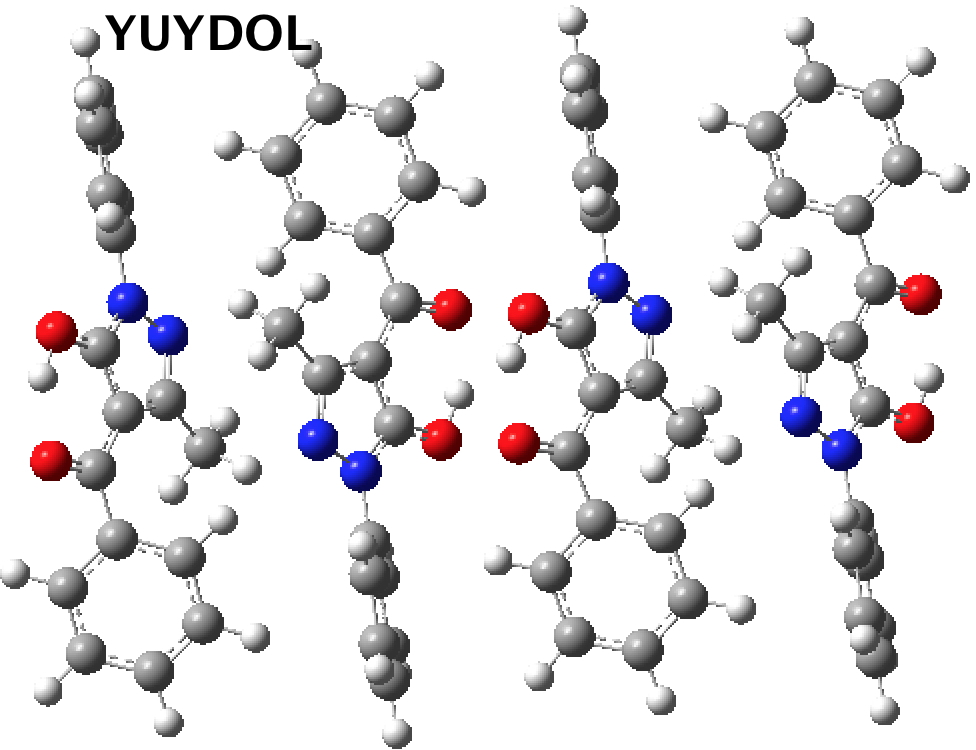
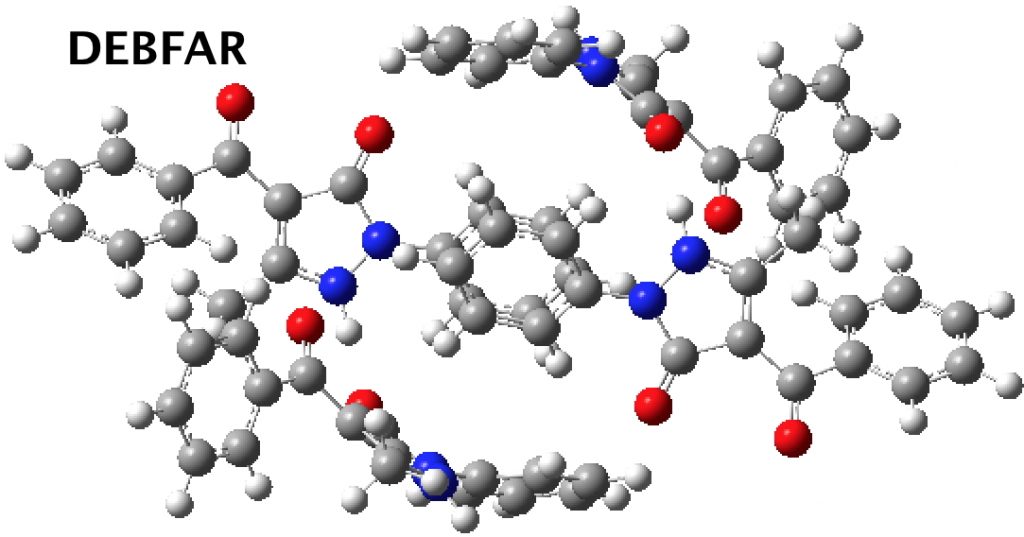
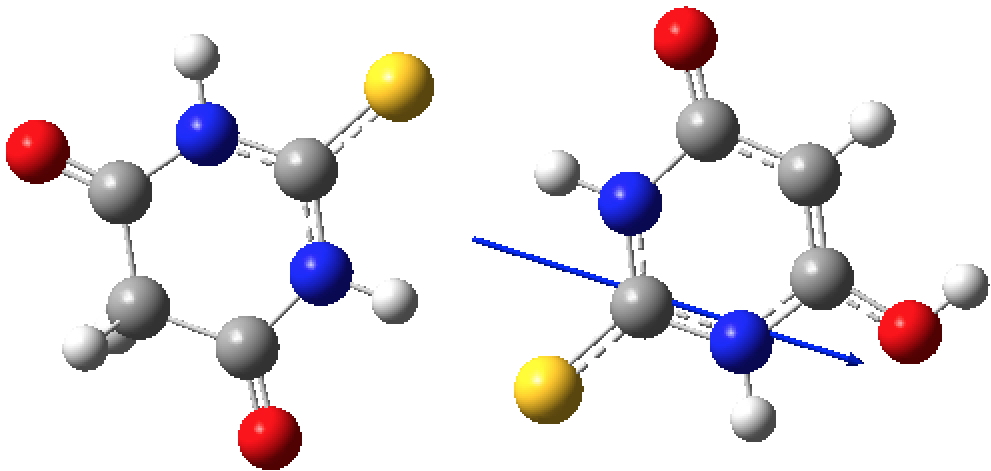
This does occur with a second example. DEBFAR[3] represents the keto form on the right which crystallises from methanol, whilst YUYDOL as the enol form on the left crystallises from n-hexane.
Calculations shed some light on this behaviour. DEBFAR has a computed (DOI: 10.14469/hpc/2591) dipole moment of 11D, whereas YUYDOL (DOI: 10.14469/hpc/2590) is 2.5D. In chloroform solutions (~half way between the two solvent polarities), the keto form is ~6.1 kcal/mol lower in ΔG than the enol. The crystal packing for the two forms is very different and the differences in this packing must clearly amount to >6.1 kcal/mol to over-ride the lesser stability of DEBFAR in solution.
The final example [4] is illustrated using scheme 2 from that article, one entitled tautomeric species of 4-hydroxynicotinic acid:
The original diagram has two unfortunate bond errors which are NOT reproduced above (and which perhaps are a good topic for discussion in tutorials with students), along with an unusual interpretation of the term tautomerism. The blue arrows above are mine and I suggest the isomerism between the connected species is resonance isomerism, and not tautomerism. So three possible different true tautomers then. Five crystal structures are reported which I list below.
- 10.5517/cctswjz (KUXPUP, 4-oxo-1,4-dihydropyridine-3-carboxylic acid, no H2O), 10.5517/ccdc.csd.cc1kfyxv (KUXPUP01 no H2O) and 10.5517/ccdc.csd.cc1kfyzx (KUXPUP02 no H2O)
- 10.5517/ccx59s4 (AVEMUK, 4-Oxo-1,4-dihydropyridine-3-carboxylic acid hemihydrate) and 10.5517/ccdc.csd.cc1kfz21 (AVEMUK01)
- 10.5517/ccdc.csd.cc1kfz54 (AKIHIN, 4-hydroxypyridin-1-ium-3-carboxylate monohydrate)
- 10.5517/ccdc.csd.cc1kfz10 (AKIHAF, 4-hydroxypyridin-1-ium-3-carboxylate)
KUXPUP and AVEMUK differ only in the presence of one solvent water molecule and both represent tautomer 2 above. AKIHIN and AKIHAF similarly represent tautomer 3 above; both are represented as 3a in the CSD and not as 3b. There are no examples of tautomer 1 in the crystal structure database; it may only exist in the gas phase. So the equilibrium 2 ⇌ 3 is another genuine example of tautomeric polymorphism, with the keto form favoured by more polar solvents, as was noted for the previous example.
With this last article,[4] comprehensive calculations at a good level were reported, including modelling the periodic cell using the Crystal program and including corrections such as BSSE (basis set superposition error) and dispersion terms. I was hopeful that this might lead me to something as simple as the computed dipole moments of the (isolated) species (as I reported above for the previous system), but these were not mentioned in the text of the article. Unfortunately, the supporting information also had no details of any such calculations, which left me frustrated again at how difficult it can be in (it has to be said) the vast majority of articles which report calculations to get details of such calculations.
Tautomeric polymorphism remains a very rare phenomenon. SciFinder for example only has 19 references citing it (2 of which are to conference talks). Perhaps the most intriguing[5] claims that 2-thiobarbituric acid has the richest collection of tautomeric polymorphs with five. Since no calculations are reported there, I might try these out and report back here.
Postscript: Here is some analysis of 2-thiobarbituric.
- THBARB (DOI 10.5517/cctbxcd, 10.5517/cctbxfg and 10.5517/cctbxgh) are three polymorphs of the keto tautomer, the isolated molecule having a small calculated dipole moment (DOI: 10.14469/hpc/2632).
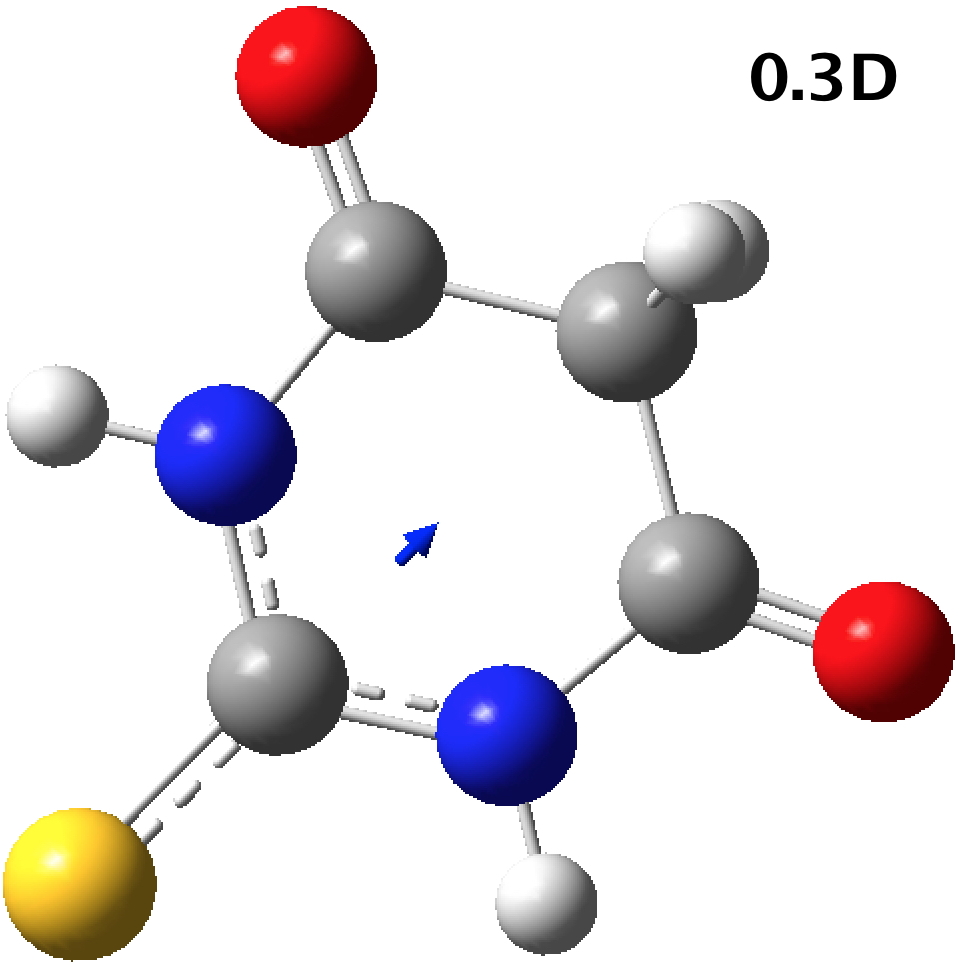
- PABNAJ (DOI: 10.5517/cctbxbc) is a polymorph in the enol form, with a much larger calculated dipole moment (DOI: 10.14469/hpc/2633)
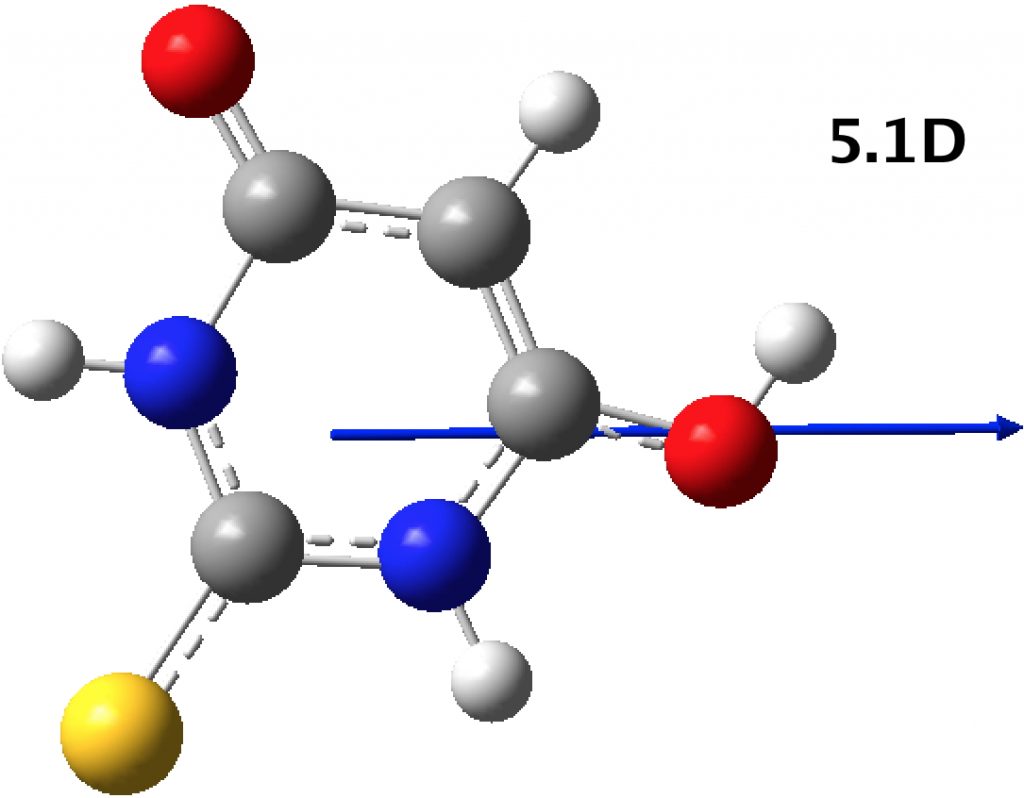
- PABNIR (DOI: 10.5517/cctbxdf) is a mixed polymorph with one enol paired with one keto form.
The relative free-energies of the isolated molecules are 0.0 (keto) and 9.0 (enol). The keto-enol pair is 0.4 kcal/mol more stable than the isolated components. This again shows the effect that crystal packing can have on the relative energies and also shows that a simple inspection of the dipole moment may cast light on the polymorphism.
References
- G.J.O. Beran, I.J. Sugden, C. Greenwell, D.H. Bowskill, C.C. Pantelides, and C.S. Adjiman, "How many more polymorphs of ROY remain undiscovered", Chemical Science, vol. 13, pp. 1288-1297, 2022. https://doi.org/10.1039/d1sc06074k
- P.M. Bhatt, and G.R. Desiraju, "Tautomeric polymorphism in omeprazole", Chemical Communications, pp. 2057, 2007. https://doi.org/10.1039/b700506g
- Y. Akama, M. Shiro, T. Ueda, and M. Kajitani, "Keto and Enol Tautomers of 4-Benzoyl-3-methyl-1-phenyl-5(2H)-pyrazolone", Acta Crystallographica Section C Crystal Structure Communications, vol. 51, pp. 1310-1314, 1995. https://doi.org/10.1107/s0108270194007389
- S. Long, M. Zhang, P. Zhou, F. Yu, S. Parkin, and T. Li, "Tautomeric Polymorphism of 4-Hydroxynicotinic Acid", Crystal Growth & Design, vol. 16, pp. 2573-2580, 2016. https://doi.org/10.1021/acs.cgd.5b01639
- M. Chierotti, L. Ferrero, N. Garino, R. Gobetto, L. Pellegrino, D. Braga, F. Grepioni, and L. Maini, "The Richest Collection of Tautomeric Polymorphs: The Case of 2‐Thiobarbituric Acid", Chemistry – A European Journal, vol. 16, pp. 4347-4358, 2010. https://doi.org/10.1002/chem.200902485
Tags: Chemistry, chloroform solutions, Conformational isomerism, Crystal, crystallography, gas phase, Ian Bruno, Isomerism, Polymorphism, Ritonavir, S-centre, Tautomer