In an earlier post, I searched for small C-C-C angles, finding one example that was also accompanied by an apparently exceptionally long C-C bond (2.18Å). But this arose from highly unusual bonding giving rise not to a single bond order but one closer to one half! How long can a “normal” (i.e single) C-C bond get, a question which has long fascinated chemists.
A naive search of the CSD is not as straightforward as it seems. Using the simple sub-structure R3C-CR3 as the search query gives LIRPEI, DOI: 10.5517/CCQ043Y[1] an apparently unexceptional molecule with a very exceptional C-C distance of 1.87Å. With long bonds one has to be ultra-careful to look at the crystallographic analysis before drawing any conclusions. One class of molecule where this has been done by many groups is the system shown below (red = long bond), with 47 entries and for which the longest C-C bond emerges with the value of 1.79Å[2]
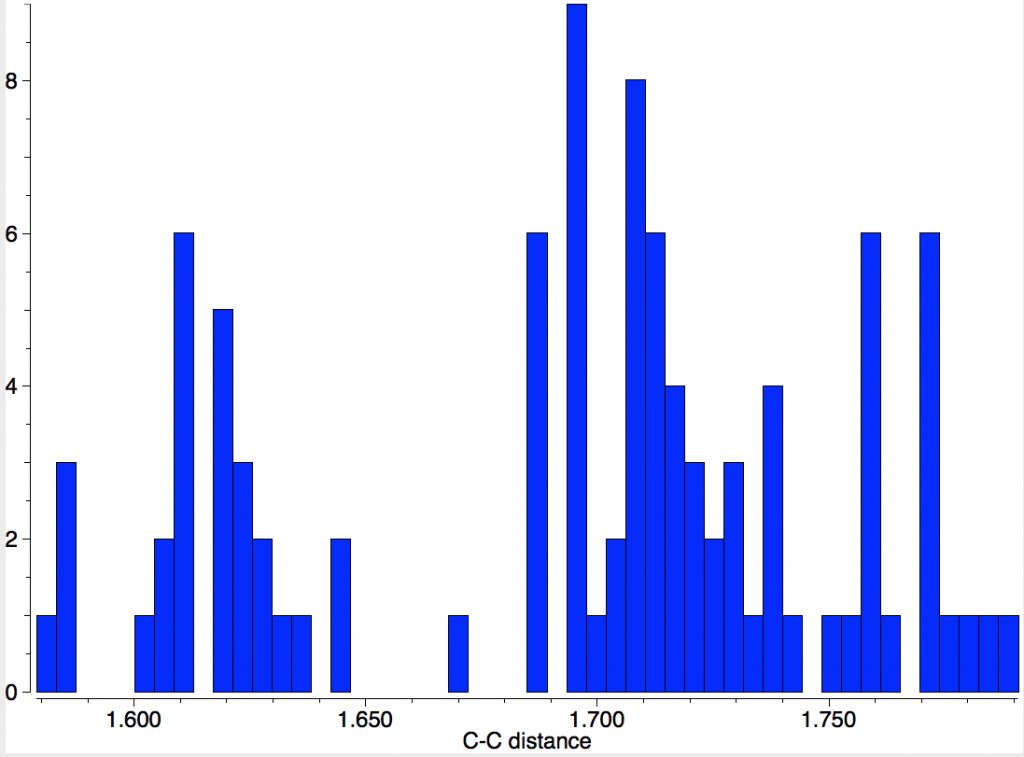
You can view this structure at DOI: 10.5517/CCS0R6Q[3] and the authors go to some pains to assure us that it is still a closed shell single bond, and not a biradical. That does seem to be the current record holder, but of course we are only talking here about molecules whose crystal structure has been determined.
I will end with an open question; how SHORT could a “single” C-C bond get? Here, a search of the CSD is entirely dominated by crystallographic artefacts, and I am not sure what the value might be.
References
- T. Takeda, H. Kawai, R. Herges, E. Mucke, Y. Sawai, K. Murakoshi, K. Fujiwara, and T. Suzuki, "Negligible diradical character for the ultralong C–C bond in 1,1,2,2-tetraarylpyracene derivatives at room temperature", Tetrahedron Letters, vol. 50, pp. 3693-3697, 2009. http://dx.doi.org/10.1016/j.tetlet.2009.03.202
Tags: Aviation, Bond order, Carbon–carbon bond, Chemical bond, chemical bonding, Interesting chemistry, naive search, search query, single bond