Allotropes are differing structural forms of the elements. The best known example is that of carbon, which comes as diamond and graphite, along with the relatively recently discovered fullerenes and now graphenes. Here I ponder whether any of the halogens can have allotropes.
Firstly, I am not aware of much discussion on the topic. But ClF3 is certainly well-known, and so it is trivial to suggest BrBr3, i.e. Br4 as an example of a halogen allotrope. Scifinder for example gives no literature hits on such a substance (either real or as a calculation; it is not always easy nowadays to tell which). So, is it stable? A B3LYP+D3/6-311++G(2d,2p) calculation reveals a free energy barrier of 17.2 kcal/mol preventing Br4 from dissociating to 2Br2.[1] The reaction however is rather exoenergic, and so to stand any chance of observing Br4, one would probably have to create it at a low temperature. But say -78° would probably be low enough to give it a long lifetime; perhaps even 0°.
So how to make it? This is pure speculation, but the red colour of bromine originates from (weak, symmetry forbidden) transitions, with energies calculated (for the 2Br2 complex) as 504, 492nm. Geometry optimisation of the first singlet excited state of 2Br2 produces the structure below, not that different from Br4.
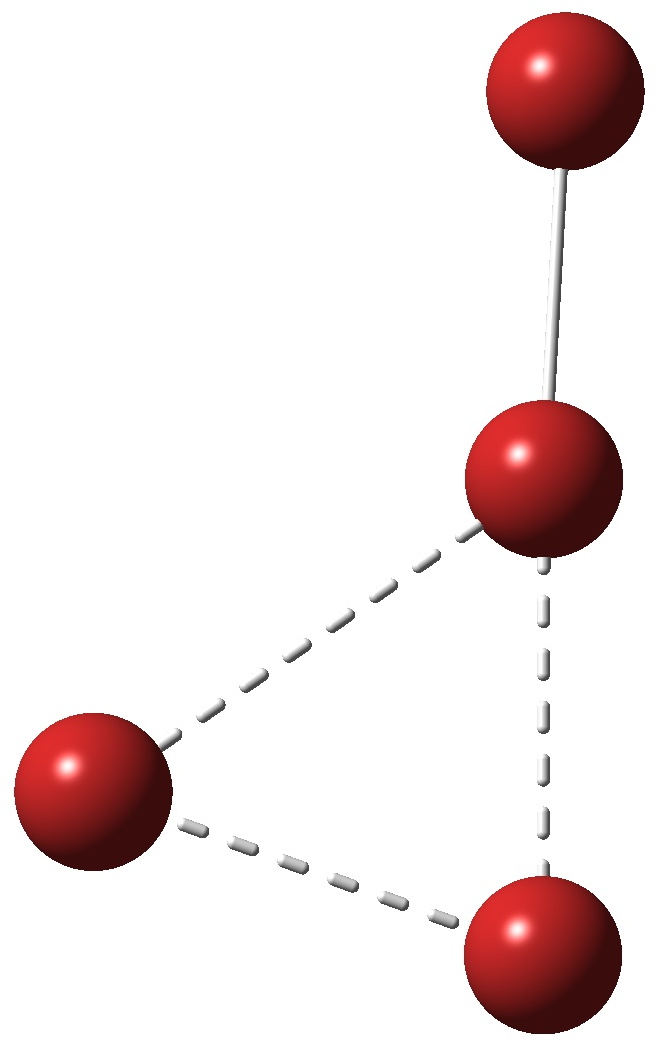
At least from these relatively simple calculations, it does seem as if an allotrope of bromine might be detectable spectroscopically, if not actually isolated as a pure substance.
Acknowledgments
This post has been cross-posted in PDF format at Authorea.
References
- Henry S Rzepa., "Br4", 2015. http://dx.doi.org/10.14469/ch/191228
Tags: Allotropy, Bromine, Carbon, Chemical elements, Chemistry, free energy barrier, Fullerene, Halogen, Hypobromite, Matter, Nonmetals, Oxidizing agents, Oxygen, pence
Peter Kenny (via Twitter), asks Iodine better able than Br to expand its valence shell and I’d guess you’d be more likely to see for I4
Actually, valence shell expansion does NOT occur in these species. For discussion of this point, see http://www.ch.imperial.ac.uk/rzepa/blog/?p=2687 There, I discuss another allotrope, this time of Iodine, I8, or I.I7, and how it is not hypervalent. Hypercoordinate yes.
In fact this allotrope of iodine is not stable, since it extrudes I2 with no activation barrier. But I4 might be.
Interesting suggestion but note that Br is a heavy element and to be sure of the reliability of ab initio calculations relativistic quantum chemical methods (DHF for instance) must be also employed. In such heavy elements it is quite probable that the effects of relativity may change even the qualitative picture emerging from non-relativistic calculations.
By the way, I am not sure to how extent chemists may share the view that a “transient” species probably only observable in very low temperatures (solid matrices?) may be called a new allotrope of an element. If this is an allotorpe then linear chains composed of carbon atoms observable in atmospheres of certain stars are also new allotorpes of carbon. Why not? I think in back of the mind of chemists there is always the assumption that an allotrope is a “stable” new molecular form of an element. By stable, of course I mean you can handle it in room temperatures and making crystals of that molecular form and things like that. Br4 hardly fits to such criteria…
Relativistic effects in Br are normally modest (for properties such as eg energies and structures, not so of course for eg magnetic shieldings), but of course this is not a state-of-the-art calculation and it would be good to know how modest they are.
I think a transient species most certainly can be useful. Singlet oxygen is a transient “allotrope” of oxygen, but useful none the less as an oxidant, as also is ozone, another allotrope. Br4 is potentially a masked Br(+).Br3(-) and so, like singlet oxygen, might be a useful source of activated Br(+).
NRT suggests that it is rather Br(-).Br3(+) than Br(+).Br3(-).
Can you expand NRT. I presume it is not the gas law nRT.
I used a Natural Resonance Theory (NRT) analysis, which is a part of the natural bond orbital (NBO) theory, to get the resonance structure weights by fitting electron density of Br4 available from the B3LYP/6-311++G(2d,2p) calculations. The Br4 structure had a D3H symmetry with the Br-Br distance of 2.5201 Å. The NRT analysis shows three dominant resonance structures Br(-).Br3(+) with identical weights 16.1*3 = 48.3%, three apparently long-bonded resonance structures (Br-Br).(Br—Br) with the weights of 8.1*3 = 24.3%, and one hypervalent structure BrBr(Br)Br, 7.9%. The natural population analysis charges and Mulliken charges predict a partial negative charge on the terminal bromine atoms of -0.13 and -0.17 a.u., respectively. The Br(-).Br3(+) structure is also consistent with the fact halogen trihalides have a less electronegative central atom.
It seems that Br4 might be detectable by microwave spectroscopy if the apparatus could withstand the corrosive power of Br2.
I have just done a search of the CSD. 155 hits are obtained for the anion Br3(-), but none for the cation Br3(+).
There are however 46 hits with R-Br(+)-R, where R tends to be C, N, Si
So on this basis alone, there is little evidence for Br3(+), or for that matter Br(+) on its own.
Re Peter’s suggestion of I4, and mindful that with this element, relativistic effects might be important, here is the calculated IRC (B3LYP/6-311++G(2d,2p), with a free energy barrier of ~13 kcal/mol. This is rather less than with Br4. Certainly worth checking what effect relativistic corrections would have on this value.
Re relativistic corrections for eg I4. Calculating the energies (no geometry re-optimisations) with inclusion of the Douglas-Kroll-Hess 2nd order scalar relativistic correction to the two-electron integrals gives an activation energy of 15.8 kcal/mol (not free energy). Although a crude estimate of the effect of relativistic corrections, this result does suggest that they are not going to be massive!
The basis set used for iodine was an all electron basis, as appropriate for making such a correction.