This is a spin-off from the table I constructed here for further chemical examples of the classical/non-classical norbornyl cation conundrum. One possible entry would include the transition state for inversion of methane via a square planar geometry as compared with e.g. NiH4 for which the square planar motif is its minimum. So is square planar methane a true transition state for inversion (of configuration) of carbon?
The history of this topic is nicely told as far back as 1993[1], when square planar methane was shown to be a 4th-order saddle point (i.e. four negative force constants) and not the first order one required of a transition state. A true transition state was located,‡ and here I show it as part of an animated IRC (intrinsic reaction coordinate). Go to DOI: 10.14469/hpc/2288 for the calculation outputs.
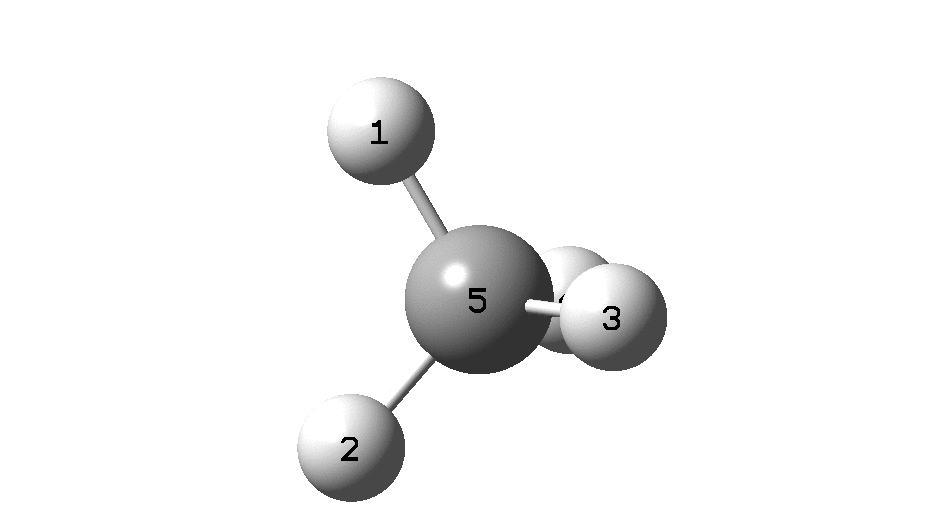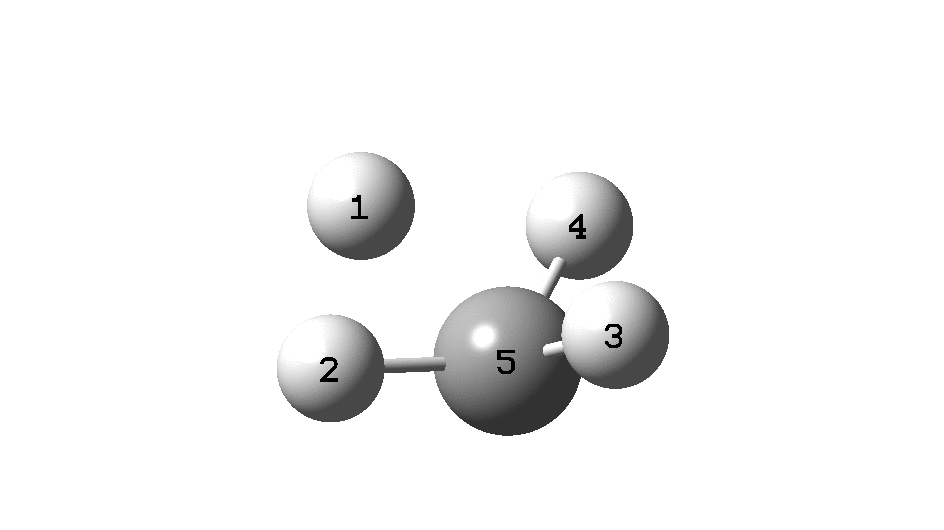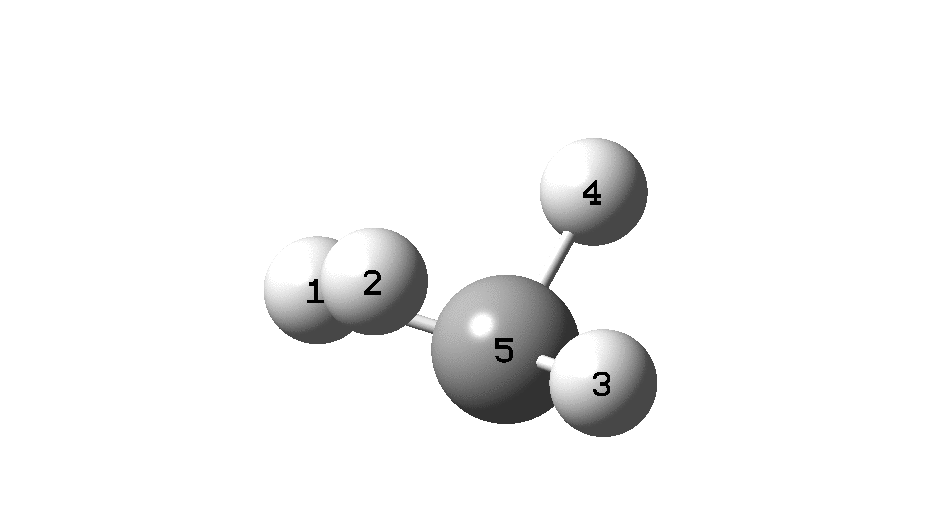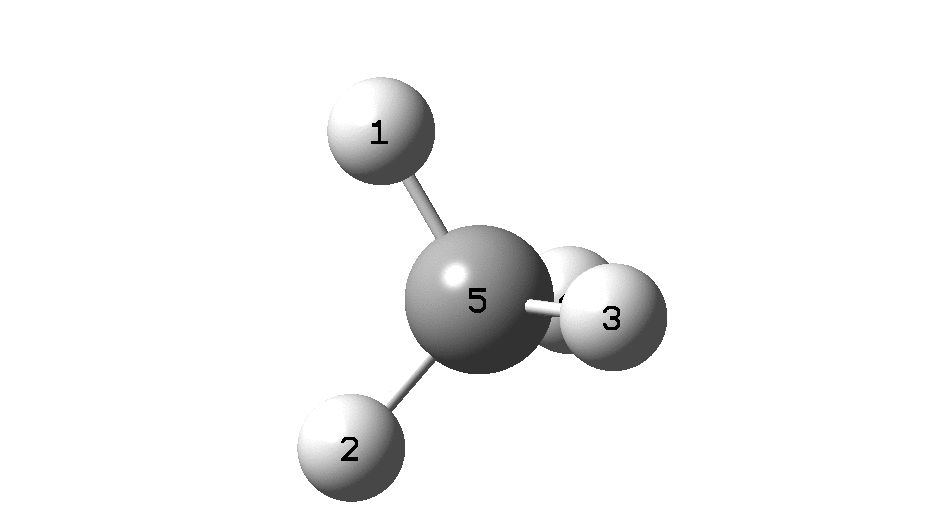
To convince yourself that the configuration really does invert, focus on the CIP rule. With atom 1 pointing behind, atoms 2 → 3 → 4 rotate in a clockwise direction. Now focus on the final point at the end of the IRC, when 2 → 3 → 4 rotate anti-clockwise. The configuration has inverted! The barrier as can be seen below is ~118 kcal/mol. At this value the half-life for the process would be far longer than the age of the universe.
The process can be described as an interesting variation on pseudorotation, for which the classic example is of course PF5.† Alternatively it can be thought of as the partial extrusion of H2 to give carbene, followed by re-addition of the H2 to reform methane. Partial because the extrusion is never fully achieved.
I have to say I did not expect anything quite so interesting to be associated with methane; one can learn from the simplest of molecules!
‡ It was not entirely trivial to recover appropriate coordinates for recomputing this TS from the article. But it is in fact an easy one to find from scratch. Hopefully with the files at 10.14469/hpc/2288 to help, this will not be an issue here.
†There are many kinds of pseudo-rotations. For others see here.[2] and [3]
References
- M.S. Gordon, and M.W. Schmidt, "Does methane invert through square planar?", Journal of the American Chemical Society, vol. 115, pp. 7486-7492, 1993. https://doi.org/10.1021/ja00069a056
- H.S. Rzepa, and M.E. Cass, "A Computational Study of the Nondissociative Mechanisms that Interchange Apical and Equatorial Atoms in Square Pyramidal Molecules", Inorganic Chemistry, vol. 45, pp. 3958-3963, 2006. https://doi.org/10.1021/ic0519988
- H.S. Rzepa, and M.E. Cass, "In Search of the Bailar and Rây−Dutt Twist Mechanisms That Racemize Chiral Trischelates: A Computational Study of Sc<sup>III</sup>, Ti<sup>IV</sup>, Co<sup>III</sup>, Zn<sup>II</sup>, Ga<sup>III</sup>, and Ge<sup>IV</sup> Complexes of a Ligand Analogue of Acetylacetonate", Inorganic Chemistry, vol. 46, pp. 8024-8031, 2007. https://doi.org/10.1021/ic062473y
Tags: Chemistry, Methane, Molecular geometry, Orbital hybridisation, Planar, Square planar molecular geometry, Stereochemistry
I noted that the inversion of methane had some carbene-like characteristics. So what if one added a substituent that staabilized a carbene. So what about the inversion of fluoromethane?
As the following IRC animation shows (DOI: 10.14469/hpc/2293), this now cleanly extrudes hydrogen to form fluorocarbene. This allows the hydrogen to essentially freely rotate and hence achieve inversion via the back reaction. So methane is unique in not fully extruding a carbene.

Here is another example the inversion of, 1,1,1-trifluoroethane (DOI: 10.14469/hpc/2302).Here, the trifluoromethyl group serves to destabilise any nascent carbene, and the reaction remains concerted.