C Shifts: Something different can occur when a carbon migrates.
The configuration at the migrating centre can be either retained (= suprafacial mode = Hückel topology) or inverted (= 1 antarafacial mode = Möbius topology). Note that Möbius transition states are relatively common in this class because there is often little strain involved in inversion of configuration at a carbon.
 Heating the reactant gave a single product Z only if the alkyl group R= t-butyl. Smaller alkyl groups gave Z along with an isomer. Z was converted to chiral ethanoic acid (along with the three other products shown) by the sequence of three reagents shown. With R=tBu only a single product is formed, whereas with smaller R groups two compounds are formed.
Heating the reactant gave a single product Z only if the alkyl group R= t-butyl. Smaller alkyl groups gave Z along with an isomer. Z was converted to chiral ethanoic acid (along with the three other products shown) by the sequence of three reagents shown. With R=tBu only a single product is formed, whereas with smaller R groups two compounds are formed.
The reason for this can be seen more clearly when quantitative models of each transition state are constructed. The Favoured form involves migration of the hydrogen suprafacially along the top face of the diene. The methyl group (on the carbon the H is leaving) points inwards in the direction of the migrating H and the R=tBu group points outwards, out of harms way. This transition state has a modelled energy ~5 kcal/mol lower than the unfavoured form, in which the H migrates (also suprafacially) along the bottom face of the diene, via a rotamer (of the C1-C2 single bond) of the original transition state, in which now the R=tBu group now points inwards, increasing the steric congestion. The result of the H migrating along the top face is to transfer the (S)-C1 chirality to (R)-C5 chirality with complete selectivity. Any migration along the bottom face would have transferred (S)-C1 chirality to form (S)-C5 instead, resulting in loss of enantiomeric (R)-purity for the ethanoic acid. It goes without saying that any contamination by antarafacial migrations of the H group would also have had this effect!
| Suprafacial/top |
|---|
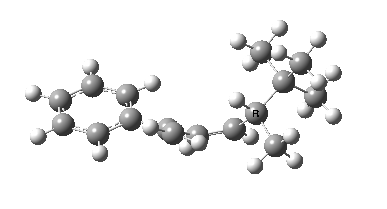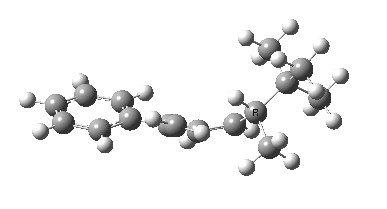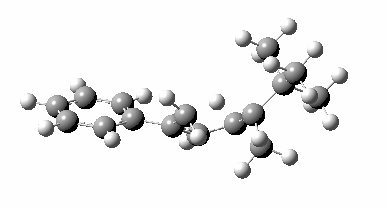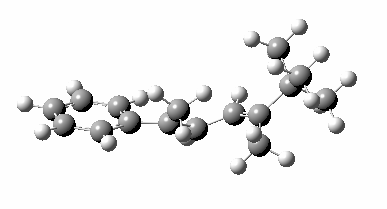 |
| suprafacial/bottom |
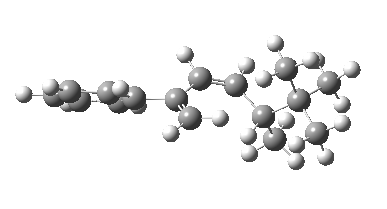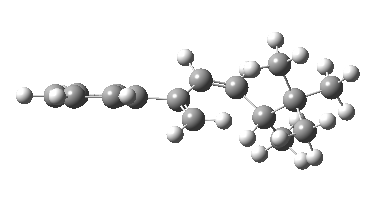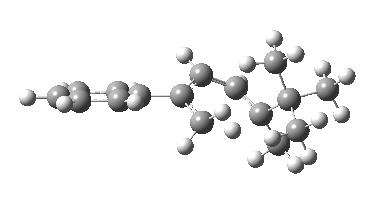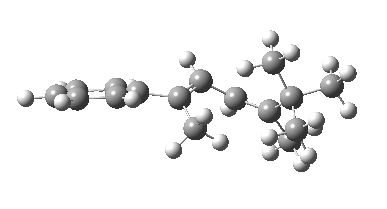 |
Another illustration of [1,5] hydrogen shifting can be seen in cyclopentadiene: The structure of this species implies that the 1H nmr spectrum should contain a triplet at about δ4 ppm corresponding to the sp3 protons and more peaks at about δ 5-6 corresponding to the sp2 protons, the ratio being 1:2.
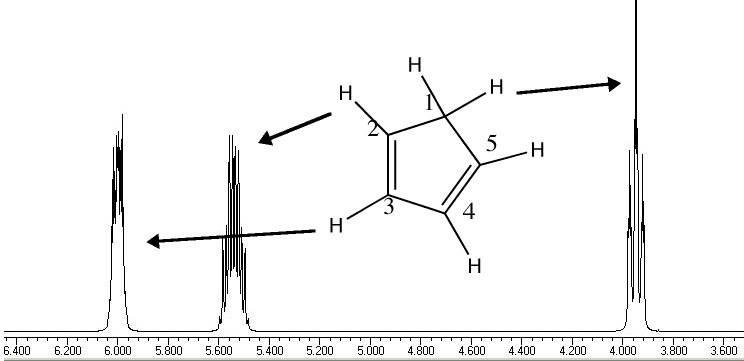
In fact, the room temperature spectrum shows only one peak at about 4.8 ppm. The reason for this is that a series of successive [1,5] hydrogen shifts is occurring so quickly that only the 'averaged' proton position is manifested in the nmr spectrum. Slowing down the exchange by cooling to about -50°C produces the expected spectrum described above! Many nmr spectra are affected by these so called 'degenerate' pericyclic processes, also called "ring whizzing".
| cationic/antarafacial | anionic/suprafacial (bottom) |
|---|---|
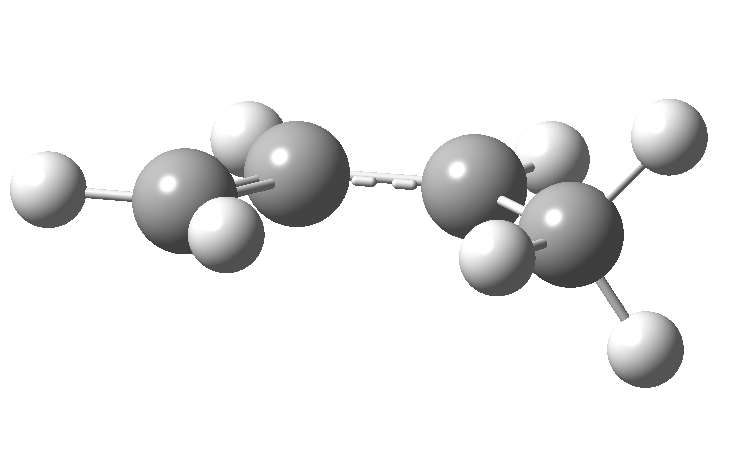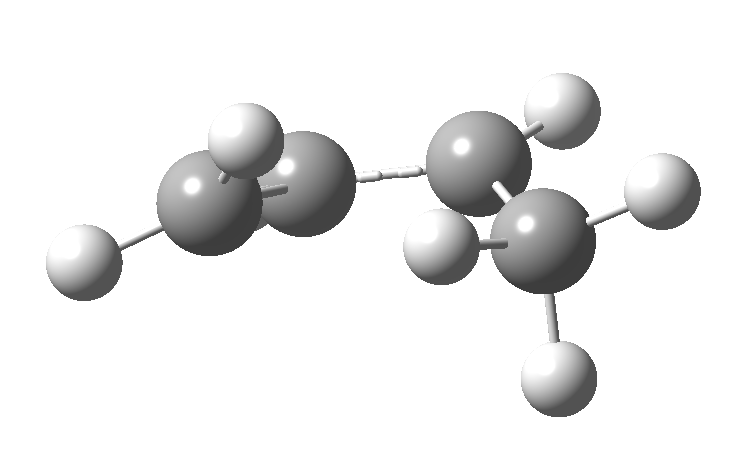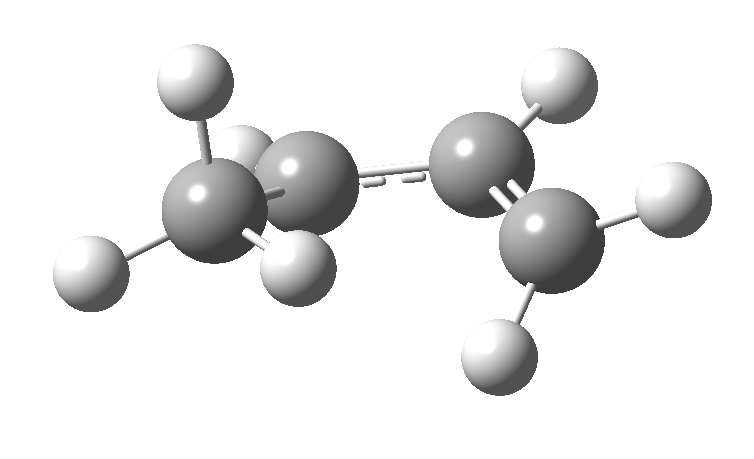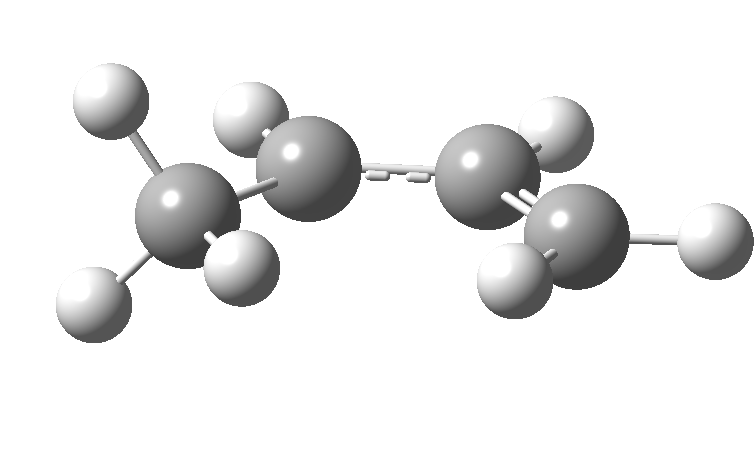 |
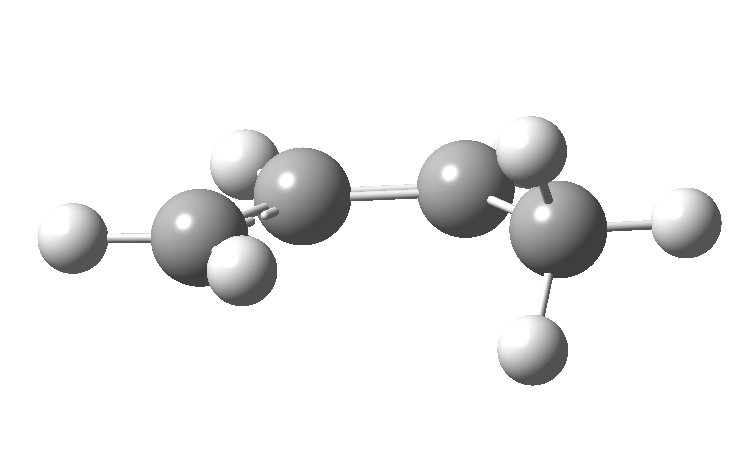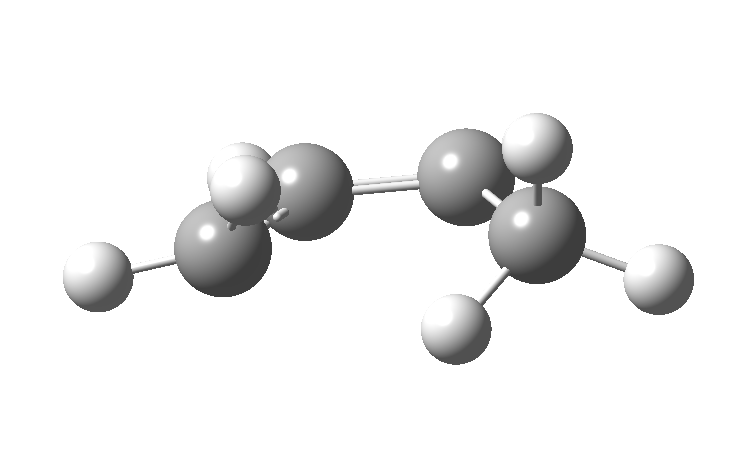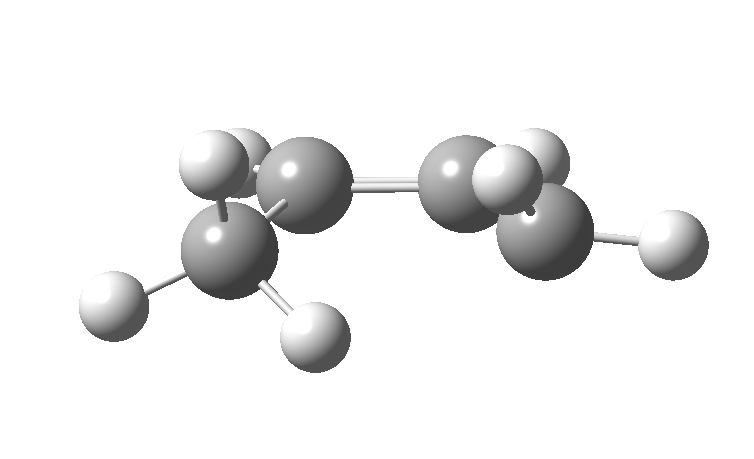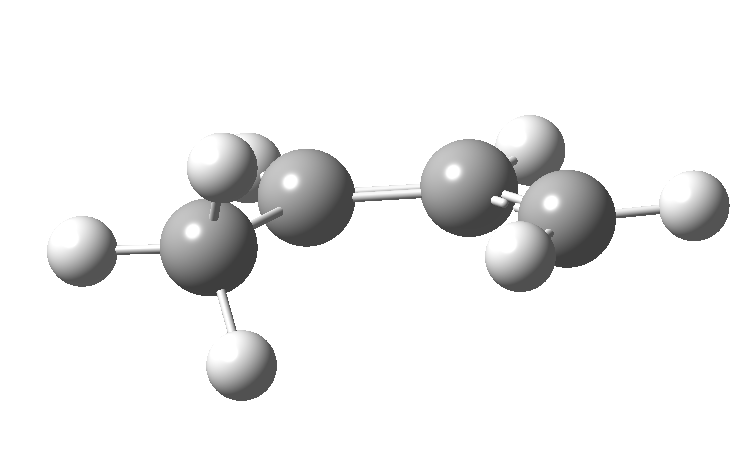 |
This involves 4 electrons, is hence a 4n process requiring Möbius topology, and this can be most easily achieved by inverting the configuration of the migrating carbon atom. If a model of this reaction is constructed, one can see that the configuration of the migrating sp3 carbon is indeed inverted.
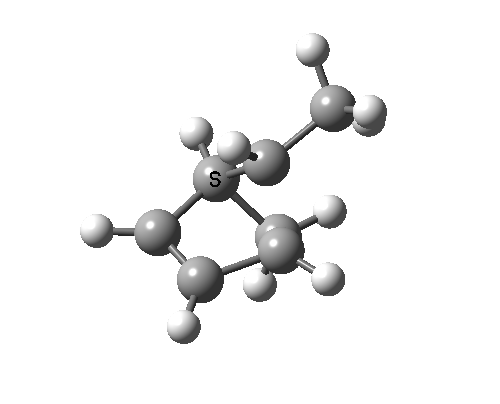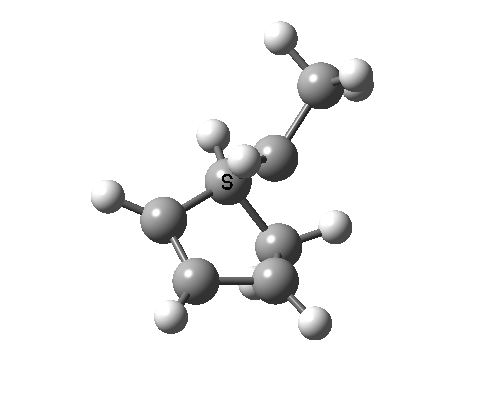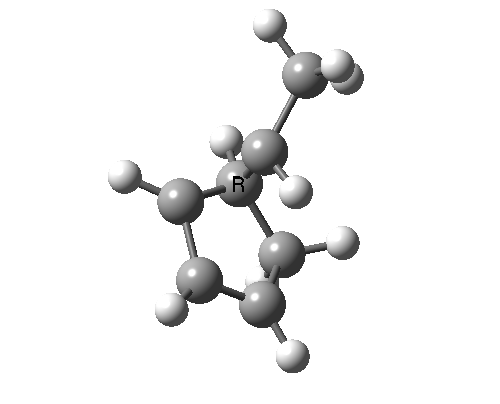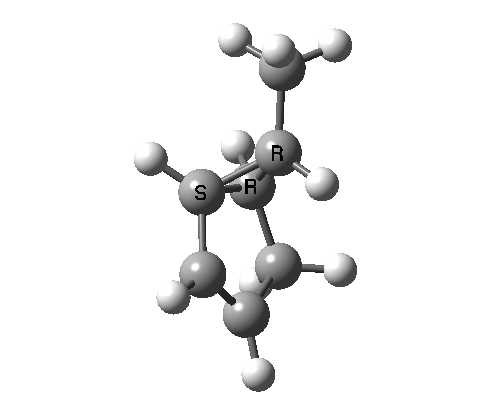
In reality, [1,3] carbon shifts may be more complex, a fair number of them proceeding via biradical intermediates, and with the stereochemical consequences controlled by reaction dynamics rather than pericyclic aromaticity. See DOI: 10.1039/b711494j
 The photochemical equivalent of the reaction above is predicted to require Hückel topology (hν/4n = supra) and the conical intersection for this reaction indeed reveals this:
The photochemical equivalent of the reaction above is predicted to require Hückel topology (hν/4n = supra) and the conical intersection for this reaction indeed reveals this:
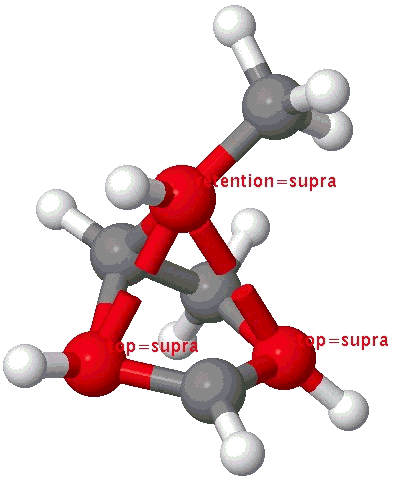
A cationic analogue, from the numbering, is classified as a [1,4] carbon migration.
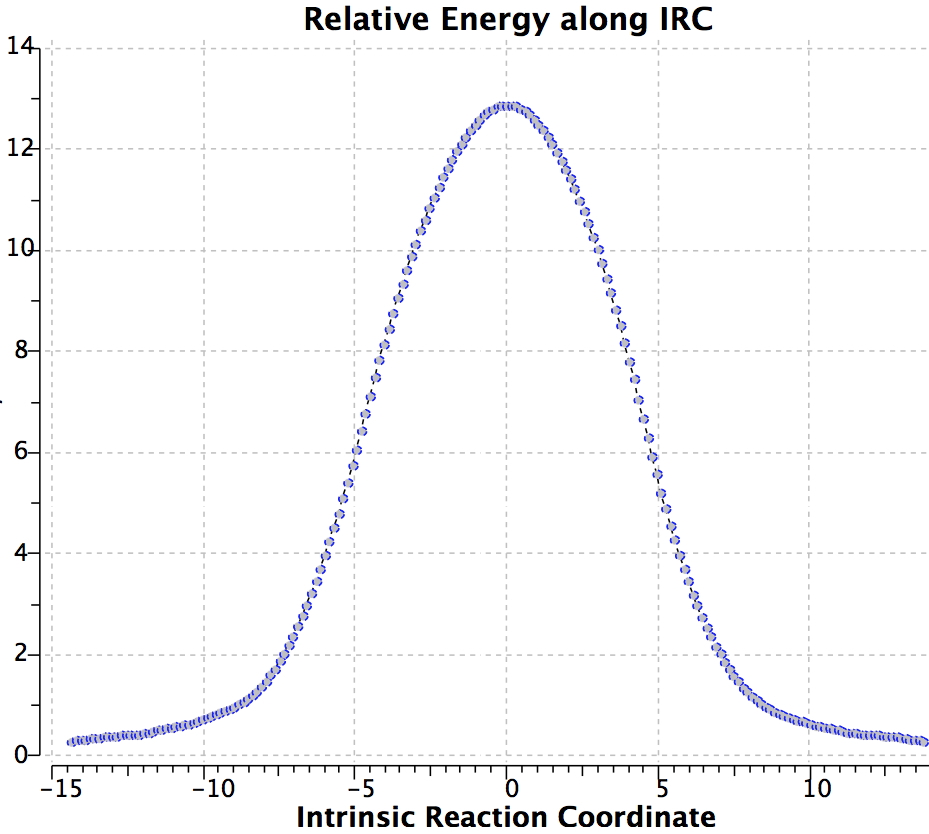
Again, the electron count corresponds to 4n, and again inversion at the migrating carbon atom is required. In this example this inversion is actually directly illustrated by inspecting the nmr spectrum (DOI: 10.1021/ja01027a059 and 10.1021/ja00827a026). Three and only three distinct methyl peaks are observed in the 1H nmr spectrum. This would only occur if [1,4] migration is fast, and also if inversion at the migrating centre were occurring, because this retains the individual identity of the two methyl groups Mea and Meb (i.e. Mea always overhangs the 5-ring). If retention at the migrating centre were to occur, the two methyl groups interchange their identity, and only two peaks would have been observed.
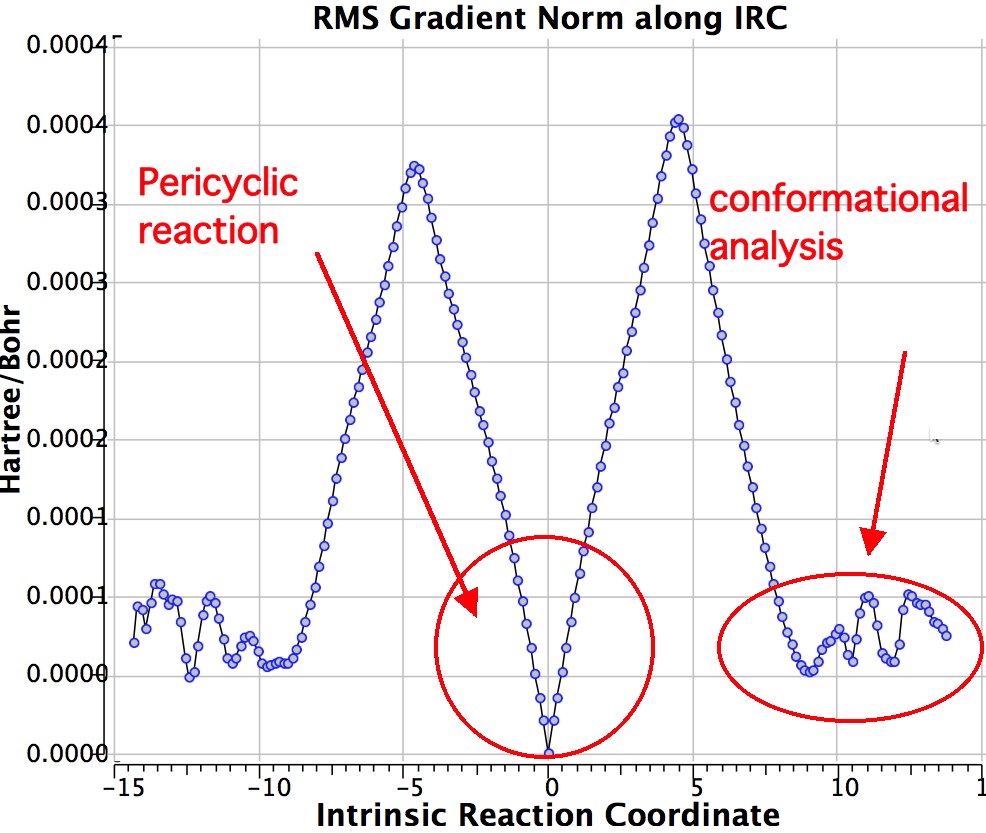
Shown below is the reaction coordinate for this methyl migration. The barrier is about 13 kcal/mol, which is what the NMR measurements indicate. However, there are two distinctly different events happening. In the region marked as IRC ± 8, we have a pericyclic reaction. In the region of IRC ± (8-15) we have a conformational process occurring. This latter will be discussed in the next lecture course! See also DOI: 10.1134/S1070428007080076
| [1,4] cationic carbon migration | The ring walk |
|---|---|
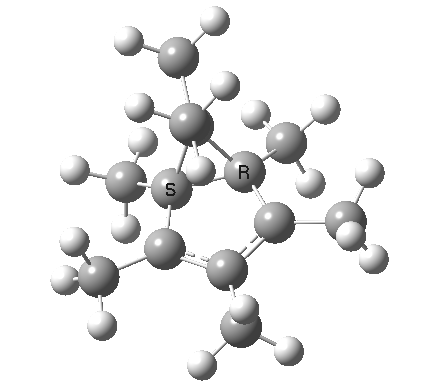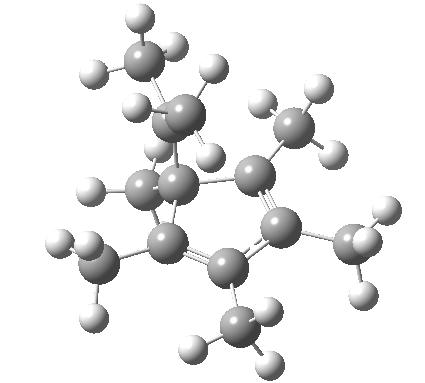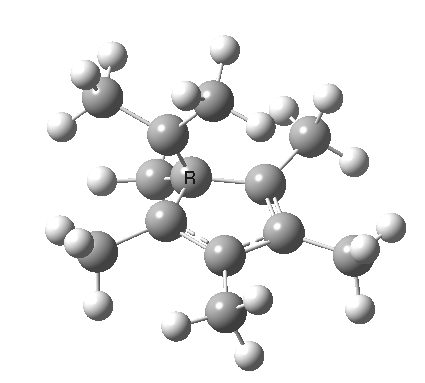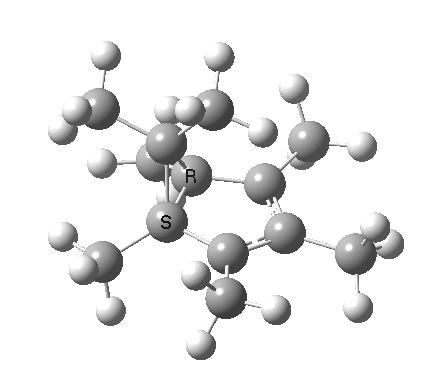 |
 |
| Thanks to Alex Genaev for the rhs image | |
| [1,6] shifts in homotropylium cation. | |
|---|---|
| Retention (barrier 17.7 kcal/mol) | Inversion (barrier 38.6 kcal/mol) |
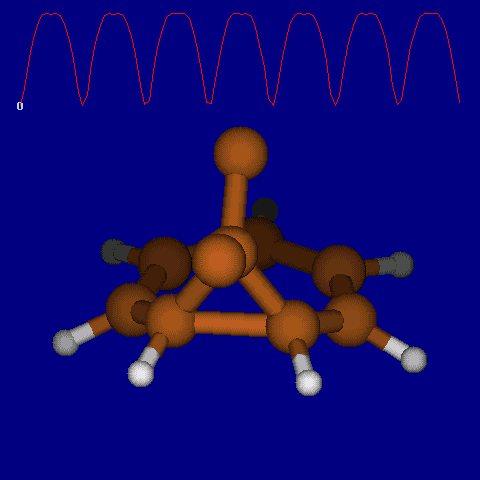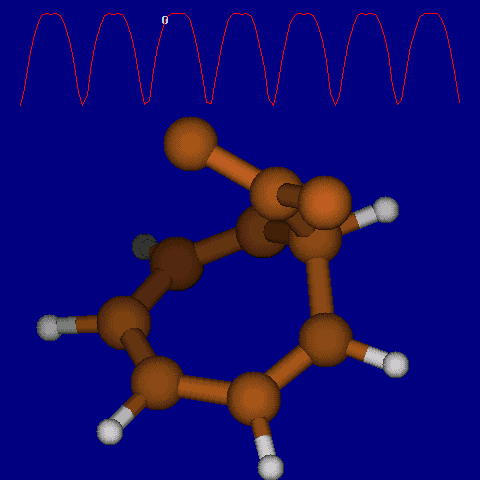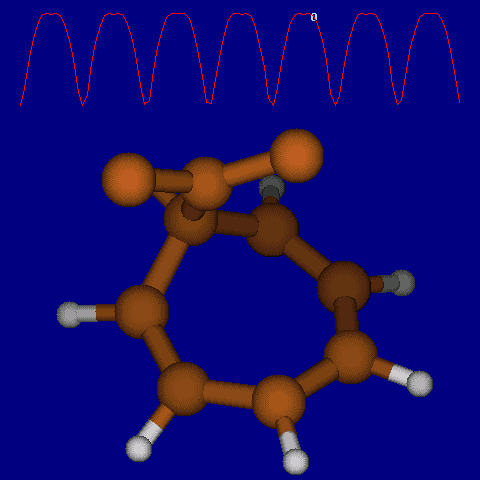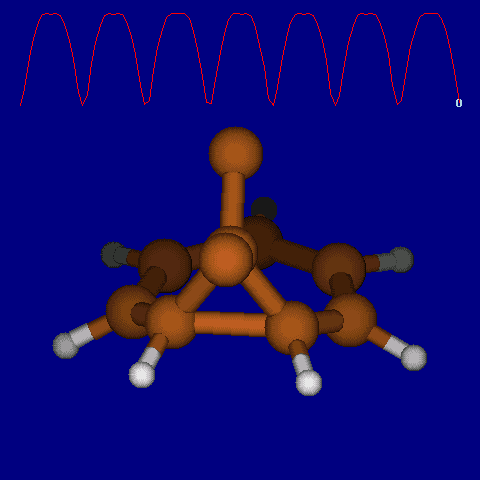 |
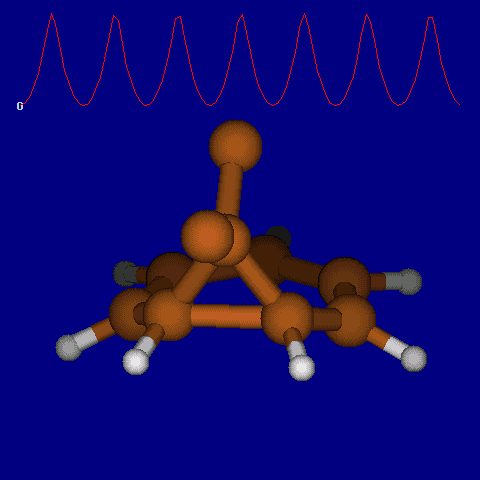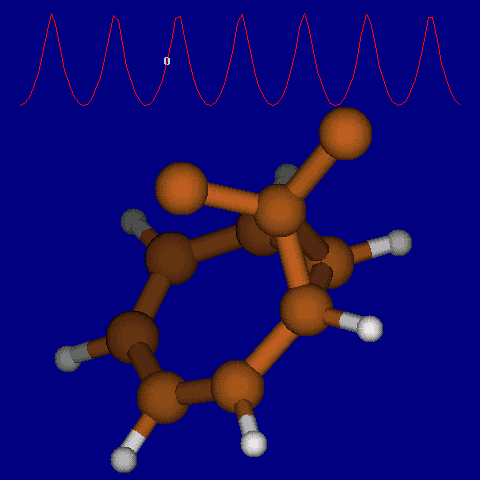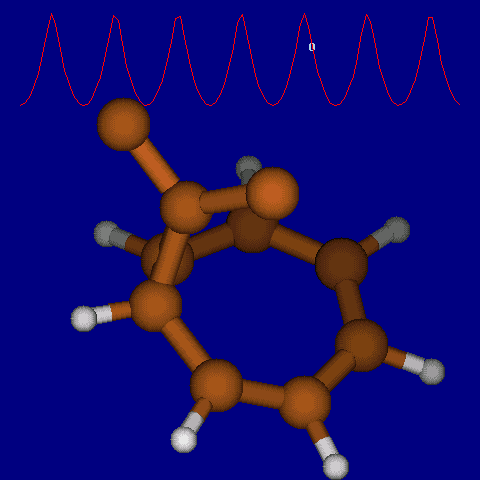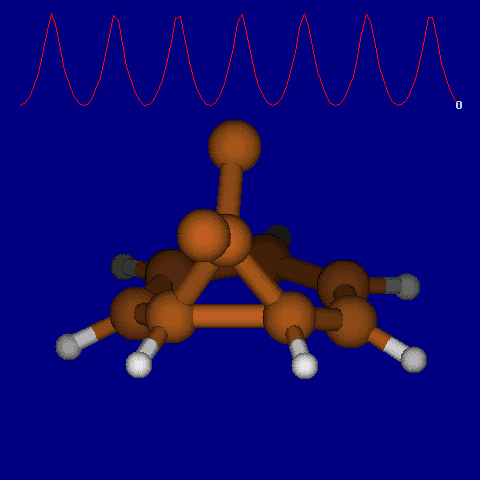 |
| Thanks to Alex Genaev for the above images. See also http://www.ch.imperial.ac.uk/rzepa/blog/?p=12852 | |
- Thus a [1,7] Hydrogen shift can only occur antarafacially (see DOI: 10.1021/ja00287a058 and 10.1021/jo00248a029).
- and shows also as [1,7] antarafacial hydrogen ring-whizzing in cycloheptatriene (the analog of the suprafacial H-whizz in cyclopentadiene)
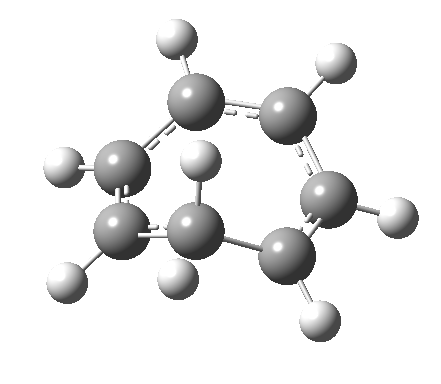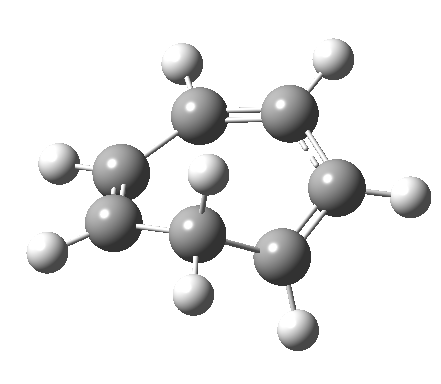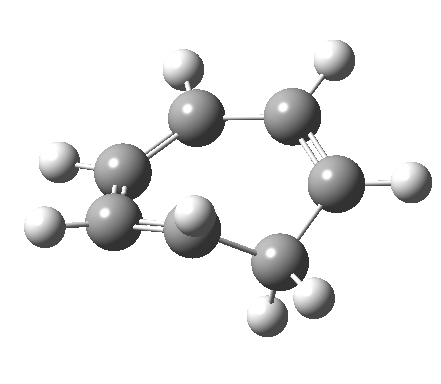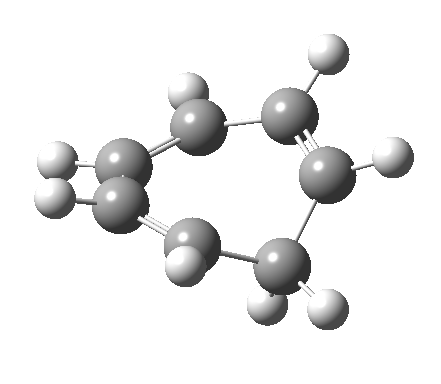 but this process competes with a
but this process competes with a - [1,5] suprafacial hydrogen shift in the same molecule, which in fact is some 40 kcal/mol lower in energy!
- A [1,7] Methyl shift can occur with antarafacial migration with retention at the methyl
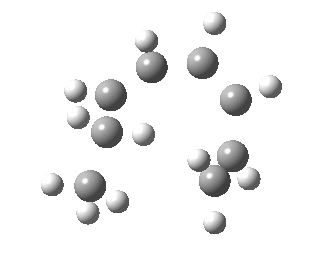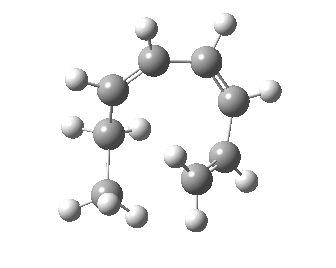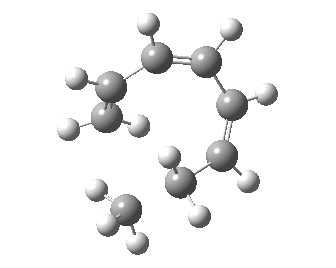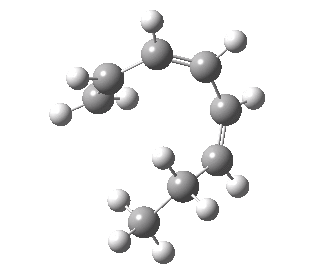
- Or with suprafacial migration with inversion at the methyl. This latter wins, being some 22 kcal/mol lower in energy.
Here, an allyl group is migrating rather than a single carbon atom. The electron count is 6 (4n+2) and the reaction is thermally allowed via Hückel topology with suprafacial components, via a chair-like transition state.
In this category thare is also a (rare) instance of the transition state being so stabilised (by aromaticity) that it becomes an intermediate instead!
When an oxygen atom is involved, the reaction is called a "Claisen rearrangement". An enzymically catalysed sigmatropic reaction involves the Claisen type conversion of "chorismate" to "prephenate" by Chorismate mutase;
 The story of frozen Cope reactions is told here. When hetero-atoms are involved, sigmatropic rearrangements involve odd numbers of atoms, as in this [2,3] rearrangement
The story of frozen Cope reactions is told here. When hetero-atoms are involved, sigmatropic rearrangements involve odd numbers of atoms, as in this [2,3] rearrangement
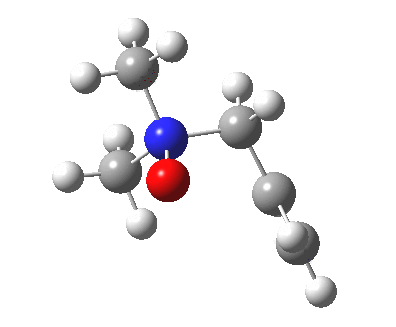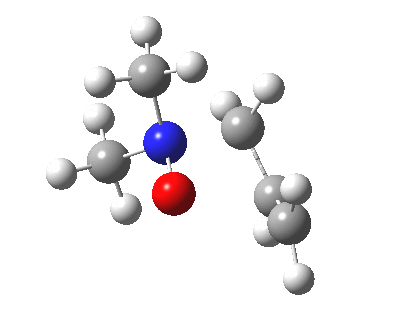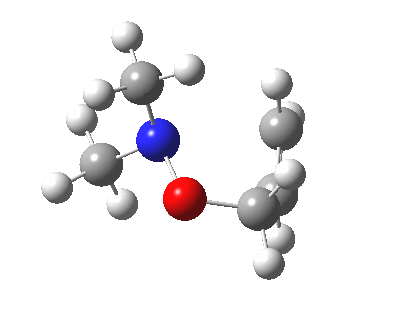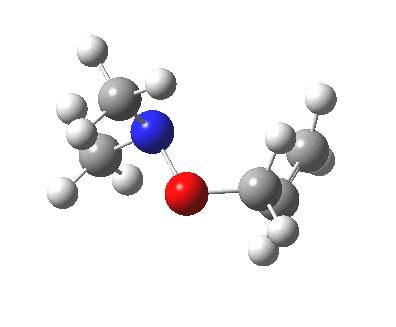
For this reaction too, an enzyme catalysed mode is known (DOI: 10.1021/ja962257w).
 The benzidine rearrangement was thought (DOI: 10.1021/ja00335a035) to proceed via a ten-electron [5,5] sigmatropic shift, suprafacially on both 5-atom groups, via a di-protonated species. The actual mechanism is now thought to be more subtle, proceeding through a transient π-complex, as first proposed by Dewar. The same mechanism also explains why PhNHOPh and PhOOPh do not exist, since the [5,5] sigmatropic has a very small barrier indeed.
The benzidine rearrangement was thought (DOI: 10.1021/ja00335a035) to proceed via a ten-electron [5,5] sigmatropic shift, suprafacially on both 5-atom groups, via a di-protonated species. The actual mechanism is now thought to be more subtle, proceeding through a transient π-complex, as first proposed by Dewar. The same mechanism also explains why PhNHOPh and PhOOPh do not exist, since the [5,5] sigmatropic has a very small barrier indeed.