Definition of Pericyclic Reactions.
A pericyclic reaction is one in which bonds are made or broken in a concerted cyclic transition state. A concerted reaction is one which involves no intermediates during the course of the reaction (left). A stepwise and therefore non-concerted and non-pericyclic reaction is shown with a discrete intermediate (right).
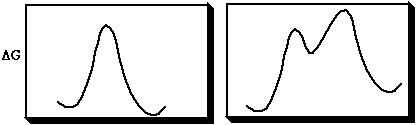
Intrinsic Reaction coordinate (IRC) → → → → → → → → →
Understanding pericyclic reactions therefore involves understanding the transition states that control them.
Pericyclic reactions have certain characteristic properties, although as usual it is not difficult to find exceptions to all these rules.
- There is no nucleophilic or electrophilic component. This means that in the arrow pushing sense, there is no beginning and no ending for the arrows, and the arrow pushing can occur in either a clockwise or anti-clockwise direction.
- Normally, no catalyst is need to promote the reactions. However, many transition metal complexes can catalyse pericyclic reactions by virtue of d-orbital participation. Lewis acids also catalyse many forms of pericyclic reactions, either directly, or by changing the mechanism of the the reaction so that it becomes a stepwise process (ie the right hand diagram above) and hence no longer a true pericyclic reaction.
- Pericyclic reactions normally show a very high stereospecificity.
- Pericyclic reactions can be frequently promoted by light (denoted hν in the text) as well as heat (denoted Δ in the text). Normally, the stereochemistry under the two sets of conditions is different, and it was (originally) thought invariably opposite. Current thinking about the photochemical route is more complex!
- Pericyclic reactions can occur in the gas phase with no solvent. There is a relatively small solvent effect on the rate of reaction (unless the reactants themselves happen to be charged, ie carbonium or carbanions). Quite recently, the use of water to accelerate pericyclic reactions (by perhaps a factor of 10 to 100) has been much investigated, but the acceleration is largely due to the formation of hydrogen bonds specific to the transition state.
- It has very recently been shown that it is possible to influence pericyclic outcomes using quite literally mechanical stress (Mechanochemistry)
- The reactions can be accelerated by the use of pressure for those involving a substantial decrease in volume. Catalysts ("molecular containers") also exist which provide suitable "cavities" for promoting pericyclic reactions such as cycloadditions.
- Unlike nucleophilic/electrophilic reactions, pericyclic reactions are unusual in that surprisingly few enzymes which catalyse them are known. Artificial catalytic antibodies ('abzymes') can be created which can perform this feat, "Diels-Alderase" may do this for cycloadditions and Chorismate mutase does do this for the Claisen rearrangement, and they act via points 5 and 7 above.
© Henry S. Rzepa, 1978-2014. Hide|show Toolbar.