Posts Tagged ‘search definition’
Thursday, June 8th, 2017
As data repositories start to flourish, it is reasonable to ask questions such as what sort of chemistry can be found there and how can I find it? Here I give an updated[1] worked example of a digital repository search for chemical content and also pose an important issue for the chemistry domain.
(more…)
References
- H.S. Rzepa, A. Mclean, and M.J. Harvey, "InChI As a Research Data Management Tool", Chemistry International, vol. 38, pp. 24-26, 2016. https://doi.org/10.1515/ci-2016-3-408
Tags:chemical content, chemical/x- media type, chemical/x-gaussian, Company: DataCite, Company: Google, digital repository search, domain-specific chemical content, media type, media:chemical/x-mnpub, media:chemical\/x\-gaussian*, Question, search definition, search engines, search string, search syntax, subject search
Posted in Chemical IT | 1 Comment »
Sunday, September 6th, 2015
An article entitled "Four Decades of the Chemistry of Planar Hypercoordinate Compounds"[1] was recently reviewed by Steve Bacharach on his blog, where you can also see comments. Given the recent crystallographic themes here, I thought I might try a search of the CSD (Cambridge structure database) to see whether anything interesting might emerge for tetracoordinate carbon.
(more…)
References
- L. Yang, E. Ganz, Z. Chen, Z. Wang, and P.V.R. Schleyer, "Four Decades of the Chemistry of Planar Hypercoordinate Compounds", Angewandte Chemie International Edition, vol. 54, pp. 9468-9501, 2015. https://doi.org/10.1002/anie.201410407
Tags:Angle, Cambridge, chemical bonding, Cycloalkane, Cyclopropane, HTML, HTML element, Ligand, metal, search definition, search results, search software, Steve Bacharach
Posted in Chemical IT, crystal_structure_mining | No Comments »
Saturday, November 1st, 2014
We are approaching 1 million recorded crystal structures (actually, around 716,000 in the CCDC and just over 300,00 in COD). One delight with having this wealth of information is the simple little explorations that can take just a minute or so to do. This one was sparked by my helping a colleague update a set of interactive lecture demos dealing with stereochemistry. Three of the examples included molecules where chirality originates in stereogenic centres with just three attached groups. An example might be a sulfoxide, for which the priority rule is to assign the lone pair present with atomic number zero. The issue then arises as to whether this centre is configurationally stable, i.e. does it invert in an umbrella motion slowly or quickly. My initial intention was to see if crystal structures could cast any light at all on this aspect.
(more…)
Tags:energy, overlap/energy match, search definition
Posted in Chemical IT, crystal_structure_mining | No Comments »
Thursday, June 26th, 2014
The Bürgi–Dunitz angle describes the trajectory of an approaching nucleophile towards the carbon atom of a carbonyl group. A colleague recently came to my office to ask about the inverse, that is what angle would an electrophile approach (an amide)? Thus it might approach either syn or anti with respect to the nitrogen, which is a feature not found with nucleophilic attack.  My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder).
My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder). 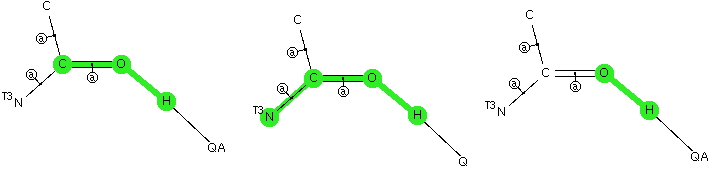 The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl)
The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl) 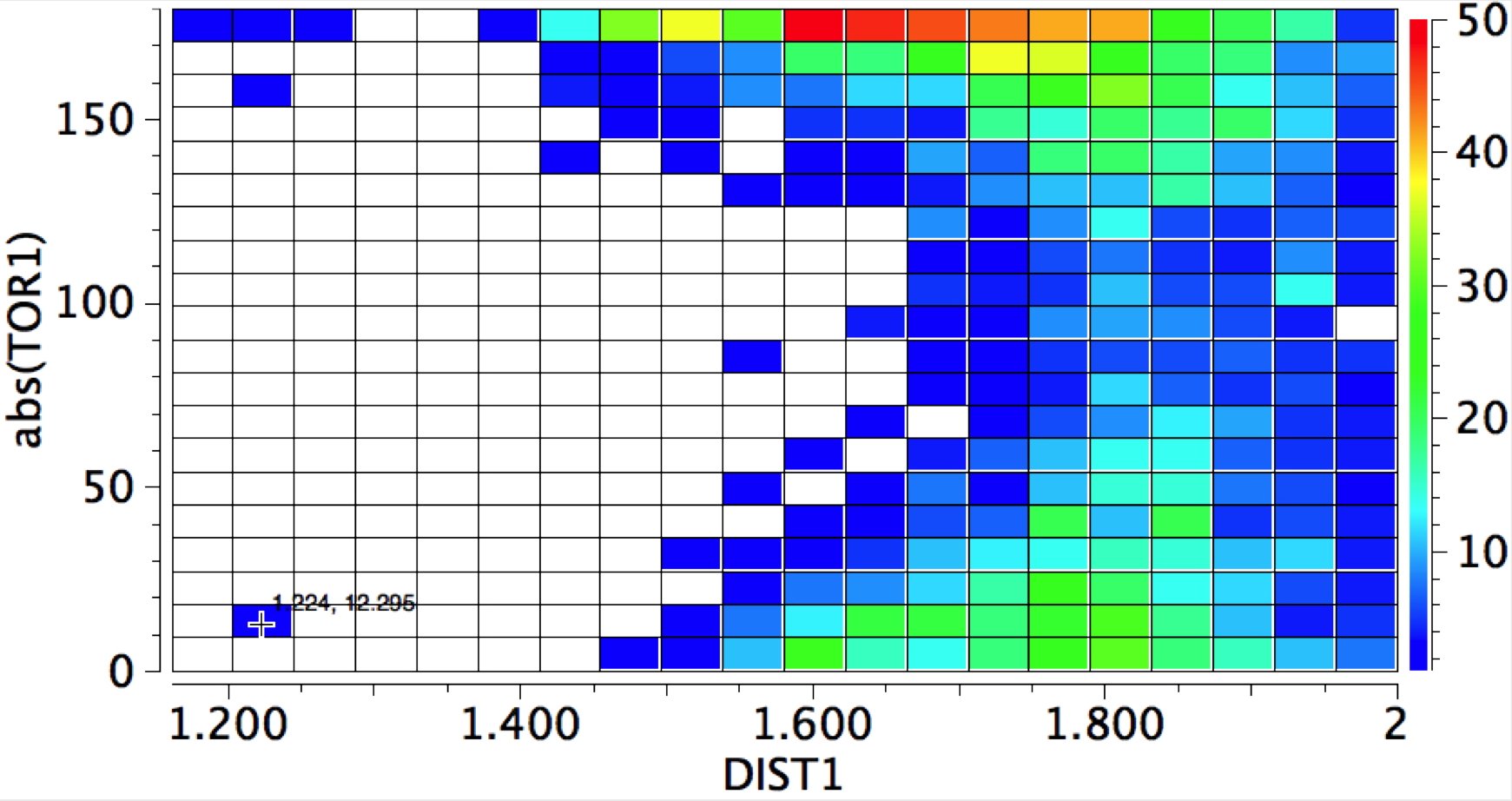
(more…)
Tags:electronics, energy, metal results, metal-π-bond interactions, search definition
Posted in crystal_structure_mining, General, Interesting chemistry | No Comments »
Wednesday, April 30th, 2014
I love experiments where the insight-to-time-taken ratio is high. This one pertains to exploring the coordination chemistry of the transition metal region of the periodic table; specifically the tetra-coordination of the series headed by Mn-Ni. Is the geometry tetrahedral, square planar, or other? One can get a statistical answer in about ten minutes.
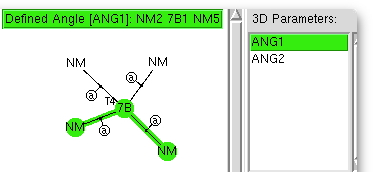 The (CCDC database) search definition required is shown above. The central atom defines the column of the period table, it is specified to have precisely four other atoms bonded to it, which can be any other element. These four bonds are specified as acyclic (to avoid any bias introduced by rings). And two angles are defined subtending the central atom. And off we go, defining on the way that the hits must be refined to an R-factor of < 0.05, have no disorder, and no errors.
The (CCDC database) search definition required is shown above. The central atom defines the column of the period table, it is specified to have precisely four other atoms bonded to it, which can be any other element. These four bonds are specified as acyclic (to avoid any bias introduced by rings). And two angles are defined subtending the central atom. And off we go, defining on the way that the hits must be refined to an R-factor of < 0.05, have no disorder, and no errors.
(more…)
Tags:data, Pt[/caption] Square, search definition, transition metal region
Posted in Chemical IT, crystal_structure_mining, General | No Comments »


