Posts Tagged ‘aromaticity’
Thursday, January 3rd, 2019
There is emerging interest in cyclic conjugated molecules that happen to have triplet spin states and which might be expected to follow a 4n rule for aromaticity.[1] The simplest such system would be the triplet state of cyclobutadiene, for which a non or anti-aromatic singlet state is always found to be lower in energy. Here I explore some crystal structures containing this motif for possible insights.
(more…)
References
- A. Kostenko, B. Tumanskii, Y. Kobayashi, M. Nakamoto, A. Sekiguchi, and Y. Apeloig, "Spectroscopic Observation of the Triplet Diradical State of a Cyclobutadiene", Angewandte Chemie International Edition, vol. 56, pp. 10183-10187, 2017. https://doi.org/10.1002/anie.201705228
Tags:antiaromaticity, aromaticity, Baird's rule, Conjugated system, crystal structure search, energy, Hückel's rule, Nature, Physical organic chemistry, Physical sciences, search query, Triplet state
Posted in Interesting chemistry | No Comments »
Sunday, March 12th, 2017
This is another of those posts that has morphed from an earlier one noting the death of the great chemist George Olah. The discussion about the norbornyl cation concentrated on whether this species existed in a single minimum symmetric energy well (the non-classical Winstein/Olah proposal) or a double minimum well connected by a symmetric transition state (the classical Brown proposal). In a comment on the post, I added other examples in chemistry of single/double minima, mapped here to non-classical/classical structures. I now expand on the examples related to small aromatic or anti-aromatic rings.
(more…)
Tags:antiaromaticity, aromaticity, Évariste Galois, Carbocation, Chemistry, equilibrium minimum energy position, George Andrew Olah, George Olah, great chemist, Jahn-Teller, minimum symmetric energy, Olah, Physical organic chemistry, Saul Winstein, symmetric energy potentials
Posted in Interesting chemistry | No Comments »
Friday, March 10th, 2017
A few years back, I did a post about the Pirkle reagent[1] and the unusual π-facial hydrogen bonding structure[2] it exhibits. For the Pirkle reagent, this bonding manifests as a close contact between the acidic OH hydrogen and the edge of a phenyl ring; the hydrogen bond is off-centre from the middle of the aryl ring. Here I update the topic, with a new search of the CSD (Cambridge structure database), but this time looking at the positional preference of that bond and whether it is on or off-centre.
(more…)
References
- H.S. Rzepa, M.L. Webb, A.M.Z. Slawin, and D.J. Williams, "? Facial hydrogen bonding in the chiral resolving agent (S)-2,2,2-trifluoro-1-(9-anthryl)ethanol and its racemic modification", Journal of the Chemical Society, Chemical Communications, pp. 765, 1991. https://doi.org/10.1039/c39910000765
- H.S. Rzepa, M.H. Smith, and M.L. Webb, "A crystallographic AM1 and PM3 SCF-MO investigation of strong OH ⋯π-alkene and alkyne hydrogen bonding interactions", J. Chem. Soc., Perkin Trans. 2, pp. 703-707, 1994. https://doi.org/10.1039/p29940000703
Tags:Ammonia, Ammonium, aromaticity, Cations, Centroid, chemical bonding, Chemistry, Hydrogen bond, Phenyl group
Posted in crystal_structure_mining | No Comments »
Wednesday, June 22nd, 2016
I previously used data mining of crystal structures to explore the directing influence of substituents on aromatic and heteroaromatic rings. Here I explore, quite literally, a different angle to the hydrogen bonding interactions between a benzene ring and OH or NH groups.
(more…)
Tags:10.1021, 10.1107, 10.5517, aromaticity, benzene, Centroid, chemical bonding, data mining, Functional groups, Hydrogen bond, Physical organic chemistry, Pyridine, Simple aromatic rings, Supramolecular chemistry
Posted in Chemical IT, crystal_structure_mining | 3 Comments »
Friday, April 17th, 2015
The knowledge that substituents on a benzene ring direct an electrophile engaged in a ring substitution reaction according to whether they withdraw or donate electrons is very old.[1] Introductory organic chemistry tells us that electron donating substituents promote the ortho and para positions over the meta. Here I try to recover some of this information by searching crystal structures.
(more…)
References
- H.E. Armstrong, "XXVIII.—An explanation of the laws which govern substitution in the case of benzenoid compounds", J. Chem. Soc., Trans., vol. 51, pp. 258-268, 1887. https://doi.org/10.1039/ct8875100258
Tags:above search, Aromatic compounds, aromaticity, Birch reduction, Chemistry, electron donating, Electrophile, Electrophilic aromatic substitution, Ether, Functional groups, little search, Organic chemistry, Physical organic chemistry, Substitution reactions
Posted in Chemical IT, crystal_structure_mining | 1 Comment »
Tuesday, September 14th, 2010
Unravelling reaction mechanisms is thought to be a 20th century phenomenon, coincident more or less with the development of electronic theories of chemistry. Hence electronic arrow pushing as a term. But here I argue that the true origin of this immensely powerful technique in chemistry goes back to the 19th century. In 1890, Henry Armstrong proposed what amounts to close to the modern mechanism for the process we now know as aromatic electrophilic substitution [1]. Beyond doubt, he invented what is now known as the Wheland Intermediate (about 50 years before Wheland wrote about it, and hence I argue here it should really be called the Armstrong/Wheland intermediate). This is illustrated (in modern style) along the top row of the diagram.
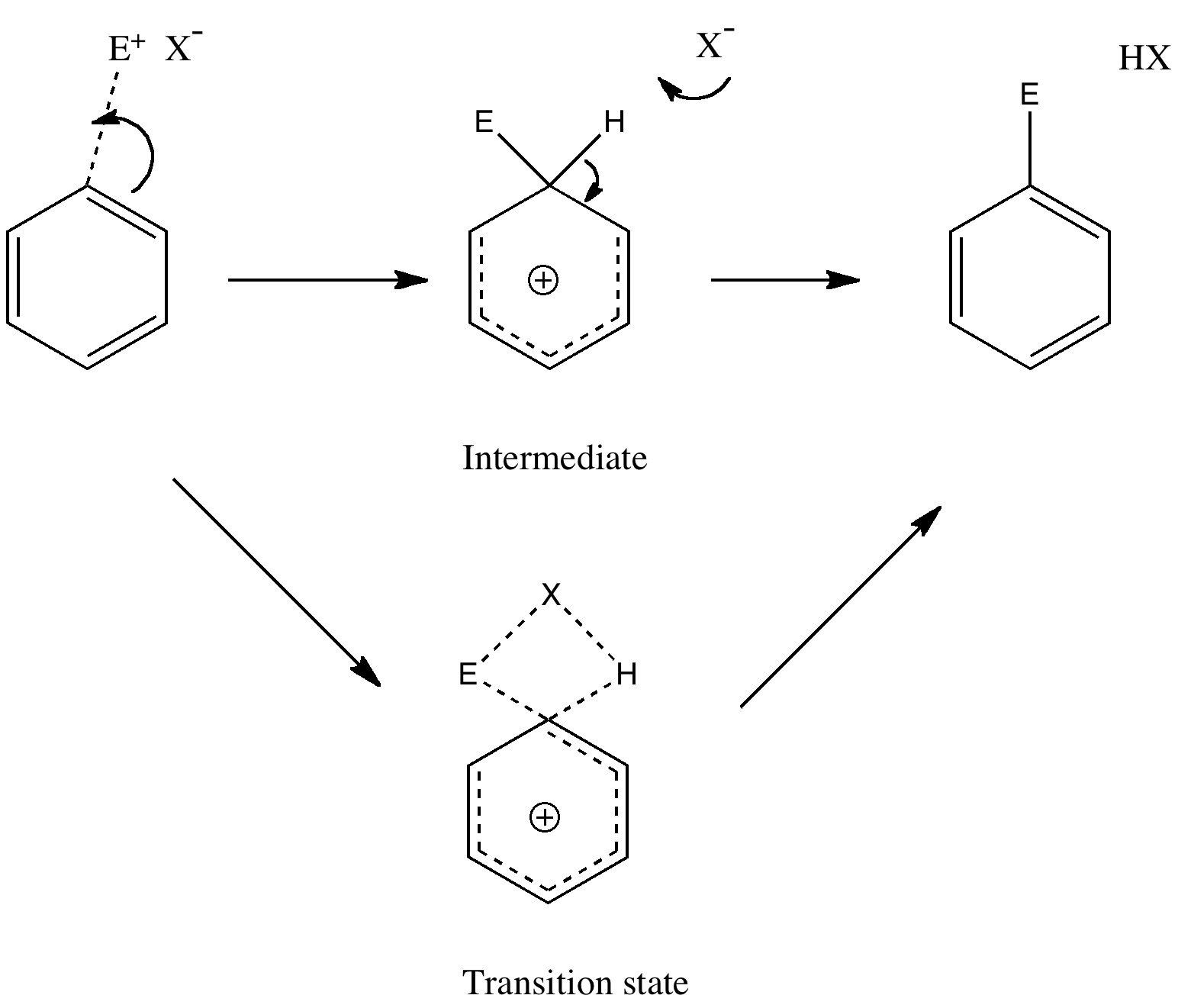
The mechanism of aromatic electrophilic substitution
(more…)
References
- "Proceedings of the Chemical Society, Vol. 6, No. 85", Proceedings of the Chemical Society (London), vol. 6, pp. 95, 1890. https://doi.org/10.1039/pl8900600095
Tags:acetic acid, animation, aromaticity, chemical, free energy, Henry Armstrong, Historical, Interesting chemistry, Wheland intermediate
Posted in Interesting chemistry | 7 Comments »
Friday, January 1st, 2010
In the previous post, the molecule F3S-C≡SF3 was found to exhibit a valence bond isomerism, one of the S-C bonds being single, the other triple, and with a large barrier (~31 kcal/mol, ν 284i cm-1) to interconversion of the two valence-bond forms. So an interesting extension of this phenomenon is shown below:
(more…)
Tags:aromaticity, Hypervalency, Interesting chemistry, pericyclic, South Carolina
Posted in Hypervalency, Interesting chemistry | 1 Comment »
Wednesday, December 9th, 2009
Clar islands are found not so much in an ocean, but in a type of molecule known as polycyclic aromatic hydrocarbons (PAH). One member of this class, graphene, is attracting a lot of attention recently as a potential material for use in computer chips. Clar coined the term in 1972 to explain the properties of PAHs, and the background is covered in a recent article by Fowler and co-workers (DOI: 10.1039/b604769f). The concept is illustrated by the following hydrocarbon:
(more…)
Tags:aromaticity, Clar, Clar islands, Cloud Clar islands, computer chips, ELF, Henry Armstrong, Interesting chemistry, non-planar systems, pence, Santos
Posted in Interesting chemistry | 1 Comment »
