This is really just a postscript to the previous post. There I showed how a search of the (small molecule) crystal database revealed the s-cis conformation about the N-C amide bond (the one with partial double bond character that prevents rotation) and how this conformation means that a C-H approaches quite closely to an adjacent oxygen. It is a tiny step from that search to a related, and very famous one named after Ramachandran[1]. Indeed this search, and the contour map used to display the results, really put crystal databases on the map so to speak.
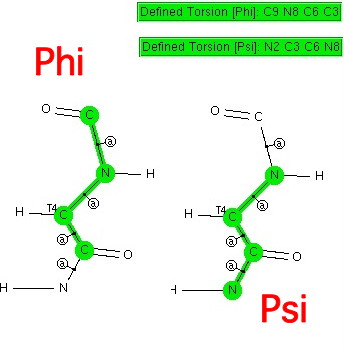
The search above defines two torsion angles about the central sp3-hybridized carbon atom (the one that makes amino acids chiral if that carbon does not carry a second hydrogen atom, i.e. glycine). The two angles are called Ψ and Φ. Such searches have been done countless times; but here I subject it to my usual constraints (R < 0.05, no disorder, no errors, T ≤ 175K and acyclic bonds along the backbone so as not to constrain it).
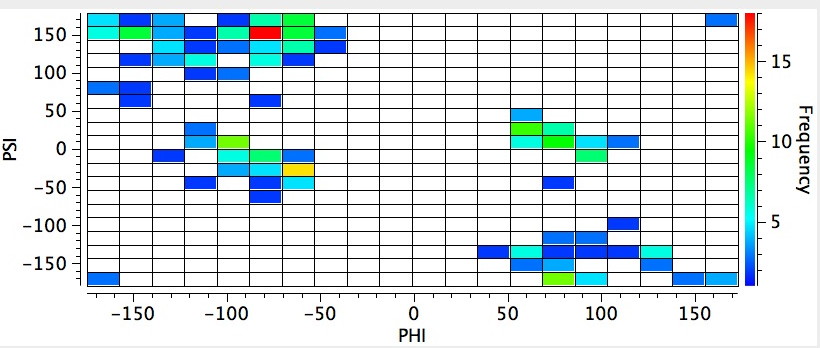
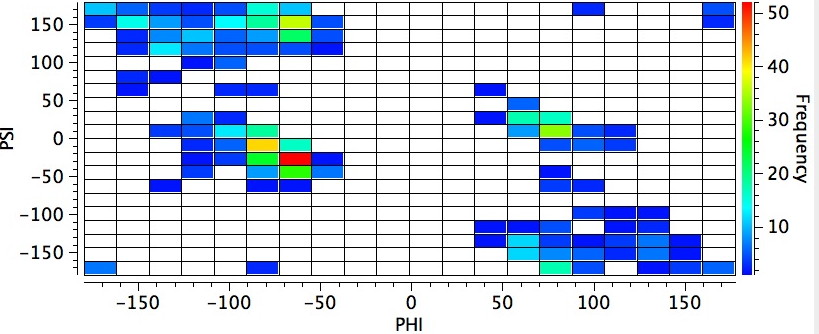
The red-spot (> 15 instances) occurs at angles of Ψ and Φ of ~ +150 and -70°. If the temperature constraint is switched off (below), a distinctly different plot emerges with the red-spot Ψ reduced to -25°. If the temperature constraint is replaced by the stipulation that the structure must be published post 2000, the first plot is largely recovered, and we may conclude that the two plots differ only because of the use of more modern diffractometers, where low temperature measurement is routine. If may also mean that more difficult structures previously inaccessible to older instruments tend to dominate the more modern plot.
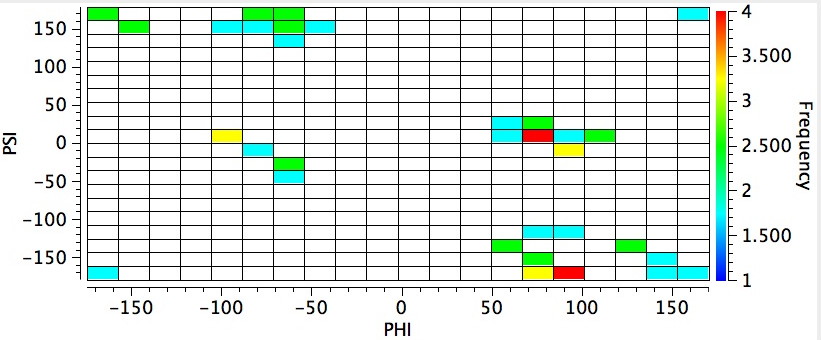
I also show a plot where the central carbon is forced to carry two H-C bonds (glycine derivatives). As is well-known, the absence of the steric bulk on that atom does induce a different backbone conformation (and hence a different location for the red spot).
And so, with this thread I have moved from discussing quite tiny molecules into the arena of much vaster biomolecules. But the basic principles are the same for both.
Acknowledgments
This post has been cross-posted in PDF format at Authorea.
References
- G. Ramachandran, C. Ramakrishnan, and V. Sasisekharan, "Stereochemistry of polypeptide chain configurations", Journal of Molecular Biology, vol. 7, pp. 95-99, 1963. https://doi.org/10.1016/s0022-2836(63)80023-6
Tags: conformational analysis, search above, Tutorial material