A game chemists often play is to guess the mechanism for any given reaction. I thought I would give it a go for the decomposition of the tris-peroxide shown below. This reaction is known to (rapidly, very rapidly) result in the production of three molecules of propanone, one of ozone and a lot of entropy (but not heat).[1]
The conventional approach might be to try to push some sensible arrows (an approach not always followed up it has to be said, by a reality check using quantum mechanics). I found the arrows that emerged from my playing interesting for the following reasons.
- One scheme might be a process involving six arrows (twelve electrons), which leads directly to the products.
- Or, one might try to group the arrows into two sets of three (shown in green and red above). A moment’s consideration suggests that the green set has to precede the red set (if not concurrent), resulting in the initial production of two molecules of propanone and the tetraoxapentane derivative shown. This new molecule then suffers a simple 2+4 pericyclic cycloelimination as the second stage.
- It is possible of course that the process may simply consist of homolytic O-O cleavages via biradicals. I will defer discussing this point until later.
The reality check would then consider whether the two processes are consecutive or concurrent. The following is computed at the wB97XD/6-311G(d,p) level, and corresponds clearly to the three green arrows shown above (no motion corresponding to the red arrows is discernible) and hence would be a consecutive process with a distinct intermediate on the path. The IRC seems to support this.
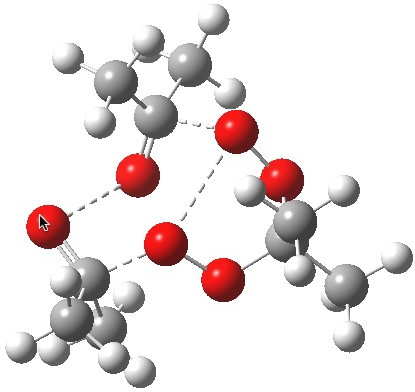 Saddle point for decomposition. Click for first imaginary vibration. |
 Saddle point for decomposition. Click for second imaginary vibration. |
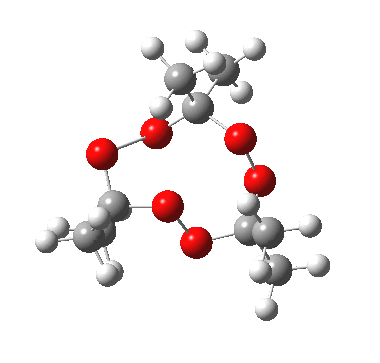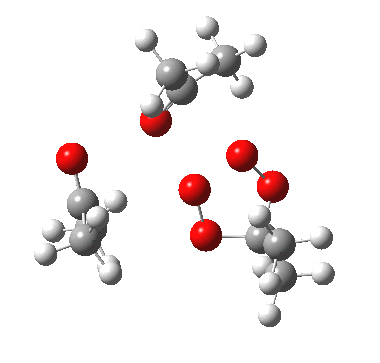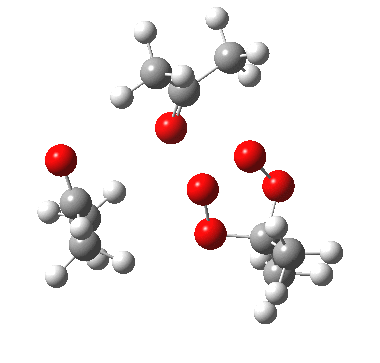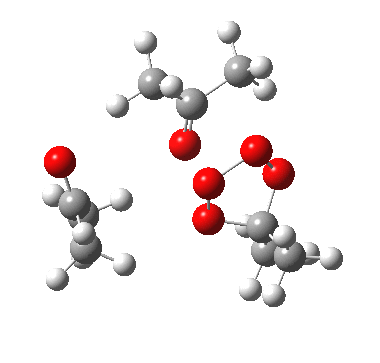 IRC for apparent concerted decomposition. |
|
The diagram is shown twice above, because this geometry is in fact not a transition state but a second-order saddle point, with two imaginary vibrations. The first corresponds to the green arrows, but the second represents an asymmetric diversion to a quite different path. This second imaginary vibration can be followed in two directions, each potentially leading to a new lower energy saddle point. I was only able to locate one of these, shown below (if I track down the other, I will append it).
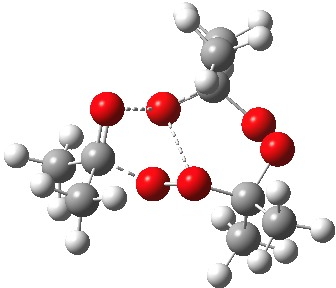 Transition state for initial fragmentation. Click for 3D. |
|
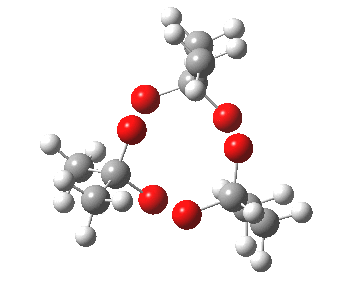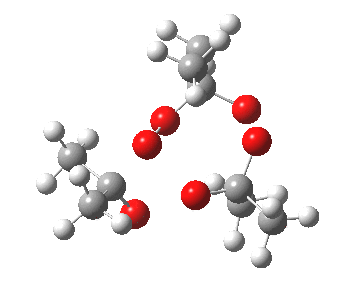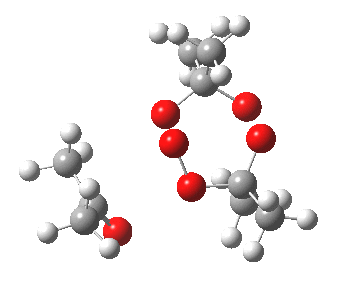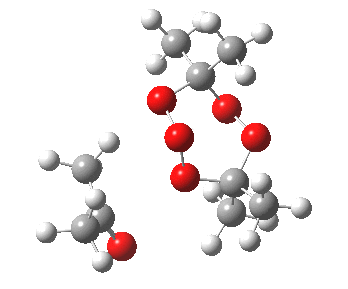 IRC for blue arrows. Click for 3D |
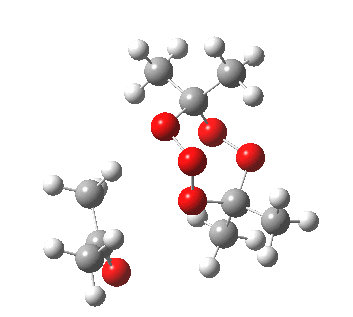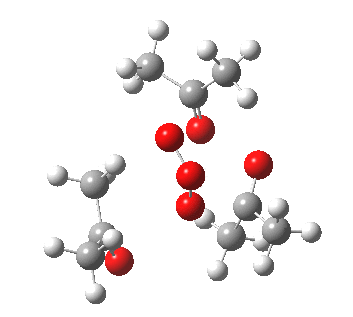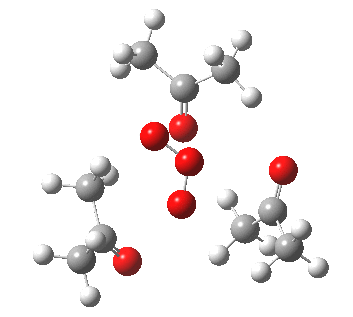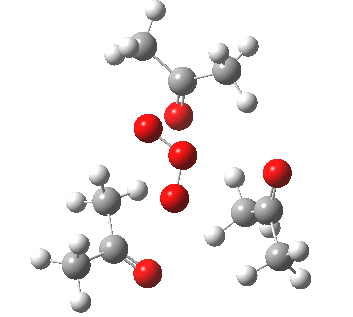 IRC for orange arrows. Click for 3D. |
As it happens, this corresponds to a rather different partitioning of the electron arrows, into a group of two first (blue) followed by four (orange). The first (proper) transition state is 5.0 kcal/mol lower in ΔG than the second order saddle point. The second transition state is 16.3 kcal/mol lower than the first. The intermediate in this process is actually different from the one shown earlier, but it can also eliminate ozone and two molecules of propanone.
What have we shown thus far? That one’s naive arrow pushing may in fact not come up with the goods. But how about that reality check? Whoops! Look at that activation barrier. The free energy (which is lower than the barrier itself because of the large +ve entropy of the reaction) is still a whopping 70 kcal/mol.
So the conclusion from all of this? Well, that homolytic pathways, involving a cleavage of an O-O bond to produce a biradical, are very probably the real mechanism after all. Something like the below perhaps? (OK, so you might have told me that at the outset!).
As milestones go, this is my 250th post.
References
- F. Dubnikova, R. Kosloff, J. Almog, Y. Zeiri, R. Boese, H. Itzhaky, A. Alt, and E. Keinan, "Decomposition of Triacetone Triperoxide Is an Entropic Explosion", Journal of the American Chemical Society, vol. 127, pp. 1146-1159, 2005. https://doi.org/10.1021/ja0464903
Tags: free energy, lower energy saddle point, Reaction Mechanism