It was three years ago that I first blogged on the topic of the Sn2 reaction. Matthias Bickelhaupt had suggested that the Sn2 reaction involving displacement at a carbon atom was an anomaly; the true behaviour was in fact exhibited by the next element down in the series, silicon. The pentacoordinate species shown below (X=Si) is naturally a minimum, and the fact that for carbon (X=C) one gets instead a transition state resulting in a significant thermal barrier (~ 20 kcal/mol) was a manifestation of abnormal behaviour.
The argument was that carbon as an atom was too small to fit snugly into a box of width ~5Å defined by the positions of two e.g. bromine atoms at more or less their closest possible approach, and instead rattled around between the two halogens, needing to surmount a barrier at the midpoint of the box. Silicon on the other hand being larger, fitted nicely into this box at the centre, and thus being unable to rattle around represented instead a minimum in the potential energy surface. I note (parenthetically) that a similar reason is often used to explain why hydrogen bonds to F are both rare and weak, whereas those to O are common and strong.
As part of a project to create a library of reaction mechanism animations, I calculated the IRC for the reaction above (X=C). This one is slightly different from those one may find in the research literature and textbooks; the counter-ion (Y=Na+) is also included so as to create a neutral system overall. The method is the usual ωB97XD/6-311+G(d,p)/SCRF=water.
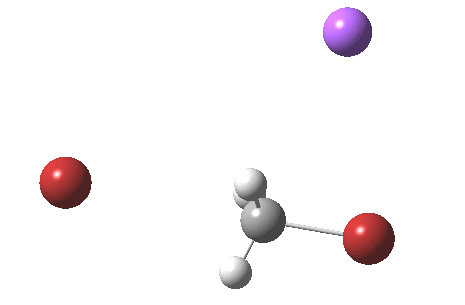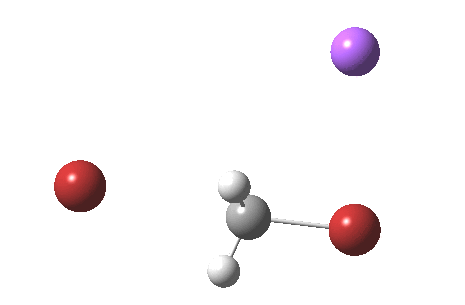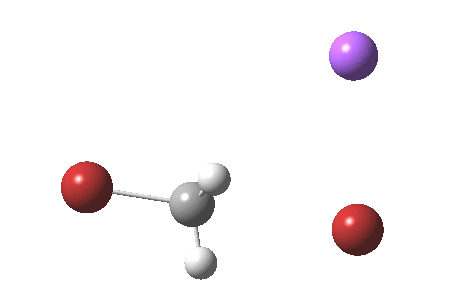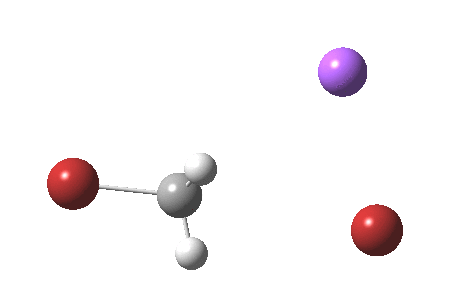
If you watch carefully, you will see that at the early and late stages of the reaction, the bromine moves, but during the middle part of the reaction both bromine atoms are absolutely stationary and it is the carbon now that adopts the motion, rattling between the two bromine atoms. This aspect can also be seen very clearly in the two plots below:
Note in particular how the gradient norm plot changes in character at IRC ± 3; the central region represents motion of carbon inside the “box”, the region outside of the box that of the bromine. I think its fascinating how such an apparently simple reaction can carry such insight into molecular behaviour.
Tags: Matthias Bickelhaupt, potential energy surface, Reaction Mechanism
Hello,
The fact that the IRC is not symmetrical may seem confusing. Do you think it is because of the Na+ position (being closer to one bromine than to the other)?
Nicolas
Yes, I deliberately did not comment on what the sodium cation was doing, wondering if anyone would spot it. It only took a few minutes for you to do so.
I actually started the TS location with the Na+ in the middle, but it gradually moved to one side, and so the system ended up unsymmetrical. I guess it gets more dipole stabilisation there than in the middle (much like the carbon in a sense also prefers an unsymmetrical equilibrium position). In this regard, it is simple to simple MO perturbation theory (the Kloopman-Salem equation), where one large and one small pairwise overlap is generally more stable than two medium overlaps.
A better model would be to include say another NaBr molecule, and perhaps a few explicit solvent molecules. Ionic reactions are certainly ones where the density of states is high; and many (almost equivalent) arrangements of the atoms are possible. So a true analysis would have to be a statistical one. But in fact, removing the Na, and computing a system with an overall charge of -1 reveals almost exactly the same behaviour, so in this case, the exact location of the counter-ion may not be crucial.
Thank you for your clarifications.
[…] Henry Rzepa Chemistry with a twist « The Sn2 reaction and the anomaly of carbon. […]