Little did I imagine, when I discovered the original example of using curly arrows to express mechanism, that the molecule described there might be rather too anarchic to use in my introductory tutorials on organic chemistry. Why? It simply breaks the (it has to be said to some extent informal) rules! Consider the dimerisation of nitrosomethane (in fact a well-known equilibrium).
Curly arrow pushing for this reaction results in two arrows heading in effect towards the same bond, at the same time! The result has two adjacent nitrogen atoms each with (formal) positive charges on them, and the N…N bond order goes from zero to two in a single step. Surely this cannot be allowed to happen as shown above? Well, the IRC (intrinsic reaction coordinate) computed from a ωB97XD/6-311G(d,p) calculation is shown below. The barrier is small, the profile uneventful. I conclude that if I ever see a student exam script showing two curly arrows heading directly towards the same bond at the same time, it might even deserve to be graded correct!
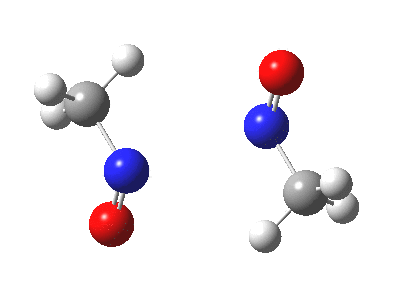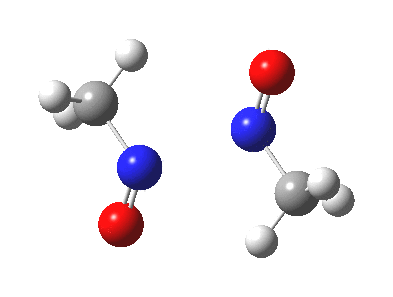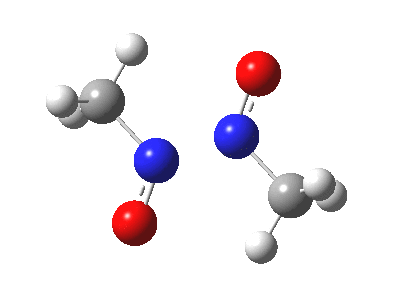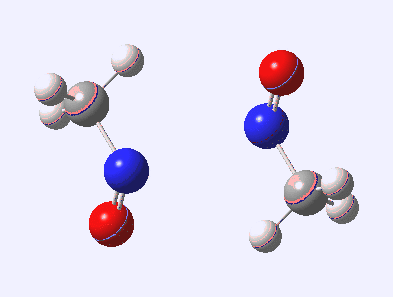
Tags: dimer, Reaction Mechanism, Tutorial material
[…] other electrophiles), why is the product p-substituted? Well, I also showed that nitrosobenzenes can easily dimerise, as shown below. This species now has a π-mesomeric resonance shown with red arrows below which […]
[…] of C1-C6 from one to two to form A. Note that we do this in two explicit steps since (with only a few exceptions), bonds rarely change their order by more than one in any distinct mechanistic […]
[…] Route B (green), overlapping the HOMO of 1 with the LUMO of 2 to create a new π-MO to be occupied by the two electrons extracted from the N-O σ-bond (a similar promotion of a σ- to a π-pair was noted in this post). […]