Streptomycin is an antibiotic active against tuberculosis, and its discovery has become something of a cause célèbre. It was first isolated on October 19, 1943 by a graduate student Albert Schatz in the laboratory of Selman Waksman at Rutgers University. I want to concentrate in this post on its molecular structure. Its initial isolation was followed by an extraordinarily concentrated period of about three years devoted to identifying that structure, culminating in a review of this chemistry in 1948 by Lemieux and Wolfram.[1] This review presents the structure as shown below (left). The modern rendering on the right is based on a crystal structure done in 1978.[2]‡
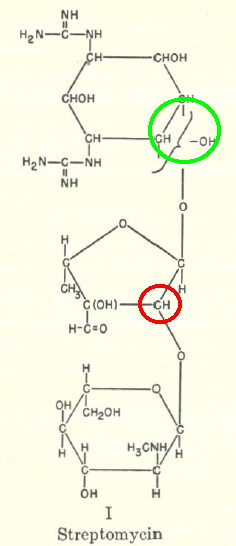 |
My interest in this was kindled by wondering how elucidating such a structure would be accomplished during the 1940s. None of the modern structural techniques were available then (NMR, MS, X-Ray); only IR and polarimetry (optical rotation). So how was it done? Well, the same way it had been done for the previous 100 years or so; degradation. In this case, into three smaller fragments, labelled A-C in the rhs diagram, and named streptidine, streptose and glucosamine in the original analysis.
- This reduction to smaller fragments is set out on page 338 of the Lemieux and Wolfram article.[1]
- The procedure to isolate streptomycin is described from p343 and the purification on p345, which concludes with the molecular formula C21H37-39N7O12
- On p346, after evaluating other methods for determining the molecular weight, C21H39N7O12 is the final candidate for its formula.
- Next, “strongly basic” degradation yields streptidine (Ring C), with the formula C8H18N6O4. Likewise, formulae were established for the other components.
- On p347, the odyssey to assemble a structure from this information begins. I will not dwell on the details. But by p359, a partial structure of Streptidine-streptose-N-methyl-L-glucosamine is suggested.
- By p366, they have boiled it down to two possibilities (they call XXXIX and XL), and they abandon the hydrolytic procedures used up to that point and adopt oxidative reactions, which narrow it down to XXXIX.
- The next property to be used to determine the structure is optical rotation. They knew it incorporates an L-sugar (whose absolute configuration was not known in 1948), and of course there are 15 stereogenic centres in the entire system (32768 possibilities). P368 -375 continues discussion of the stereochemistry, and in particular the need to demonstrate that none of the degradative/oxidative procedures have interfered with it, so to speak.
- By p375, it has all boiled down to the stereochemistry of the glycosidic bonds (marked with a red ring above). This was assigned on the basis of optical rotations and the use of additive rules (Table 1 of their article). This discussion ends with the stereochemistry shown above. Although initially assigned trans, it was subsequently revised to cis, and then back to trans again.
- On p382, the wrap up has started. Table III there shows the properties of the 18 products obtained merely from the preparation of ring A (for comparison with the product obtained by degradation). Some 127 articles have been cited for supporting information and around 50 pages of tight logical argument presented as evidence, similar in length indeed to the longer mathematical proofs!
- There was one residual uncertainty (green ring above) that had to wait for the crystal structure in 1978 to resolve. It took so long because of the challenge of finding a crystalline derivative.
I cannot help but note that the skills required to assemble a structure by degradation, and no use of NMR, MS or X-ray, were formidable, and very probably there are few chemists alive nowadays who could do a similar job (the motivation to do so would also be lacking). Assuming good crystals were available, solving such a structure nowadays using crystallography would only take 24 hours or so. And structures with 100+ stereogenic centres can now be done. When Woodward mused about the progress in chemistry, he might have had streptomycin in mind. I think it is worth remembering that the structural chemistry of 60 years ago was quite an intellectual achievement.
‡In fact the structure was first reported in 1968[3] but the coordinates in the CSD (Cambridge structure database) derive from the 1978 report.
References
- R. Lemieux, and M. Wolfrom, "The Chemistry of Streptomycin", Advances in Carbohydrate Chemistry, pp. 337-384, 1948. https://doi.org/10.1016/s0096-5332(08)60034-x
- S. Neidle, D. Rogers, and M.B. Hursthouse, "The crystal and molecular structure of streptomycin oxime selenate tetrahydrate", Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences, vol. 359, pp. 365-388, 1978. https://doi.org/10.1098/rspa.1978.0047
- S. Neidle, D. Rogers, and M. Hursthouse, "The crystal and molecular structure of streptomycin oxime selenate", Tetrahedron Letters, vol. 9, pp. 4725-4728, 1968. https://doi.org/10.1016/s0040-4039(00)75942-9
Tags: Albert Schatz, candidate for its formula, Historical, laboratory of Selman Waksman, MS, muse, pence, Rutgers University, tuberculosis, X-ray
Fascinating study! I do wonder at times about the tremendous amount of chemistry, especially related to structure elucidation, that has been lost with the advent of modern spectroscopy and crystallography. A few examples: (1) every freshman student here in the US will be able to argue at length about electron occupancy and the shape of an sp3 hybrid orbital (which is of course totally imaginary), yet probably could not tell you how to separate gold from lead (2) When I was an undergraduate student at Illinois, I recall a cume exam given to the organic grad students that was essentially walking through the procedure that Fischer used to determine the structure of the elemental sugars – how many of us can do that today? (3) A few years back a grad student complained to me that he couldn’t figure out if his compound contained a hydoxyl group or not based on his NMR. When I suggested running an IR, he was flabbergasted.
Is this always progress?