In the previous post, I wrote about the processes that might be involved in a molecular wheel rotating. A nano car has four wheels, and surely the most amazing thing is how the wheels manage to move in synchrony. This is one hell of a tough problem, and I do not attempt an answer here, but simply record an odd observation.
To rotate the wheel, the axle has to rotate, and this can be made to happen by electronically exciting it to decouple the two π-electrons. To study the excited state computationally requires a technique known as CASSCF, and the molecule above is far too big for such a method. But a crude approximation would be to decouple the spins of the two π-electrons by computing the triplet state. One can in fact decouple progressively by computing respectively the triplet, quintet, septet and nonet states. To ensure equality, these calculations were all started from the optimised singlet geometry, and allowed to evolve. One might imagine for example that the triplet state might end up delocalized over the entire molecule, weakening all four axles at the same time, although of course for this to happen, the nitrogens would have to participate.
The animation below shows the progress of the septet state. Watch the four axles individually, and notice how from the very start, three of them start to rotate, whilst the fourth (bottom left) stays rock steady!
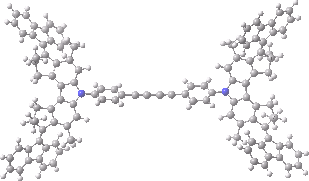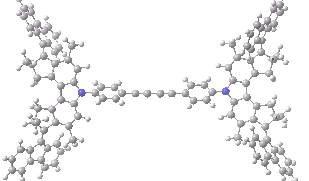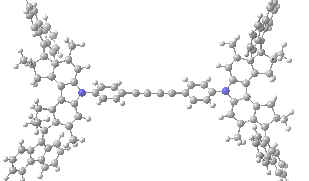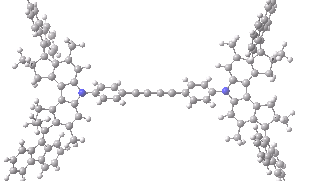
Septet spin state for the nano car. Click for 3D of final geometry.
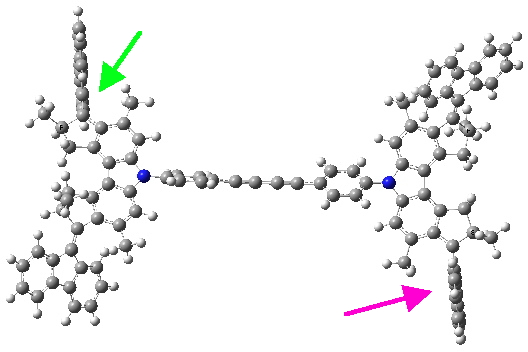
Quintet state. Click for 3D.
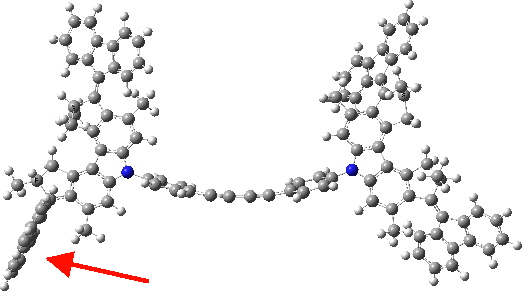
Triplet state. Click for 3D.
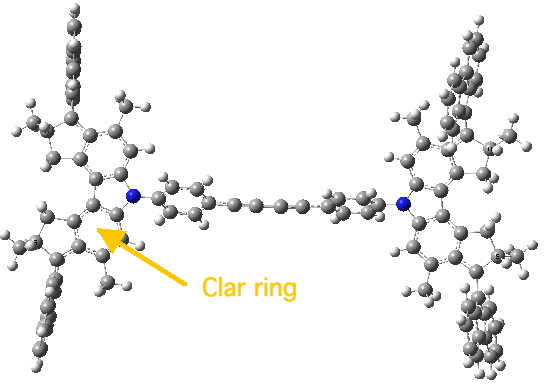
Nonet state. Click for 3D.
Which makes the ability of this nano car to coordinate the motions of its wheels when travelling even more interesting!
[…] ultimate reduction in size for an engineer is to a single molecule. It’s been done for a car; now it has been reported for the pixel […]
Ben Feringa, the principle author of the article described above, is one of the recipients of the 2016 Nobel prize for chemistry. See http://www.kva.se/en/pressroom/2016/the-nobel-prize-in-chemistry-2016/