Fascination with nano-objects, molecules which resemble every day devices, is increasing. Thus the world’s smallest car has just been built[1]. The mechanics of such a device can often be understood in terms of chemical concepts taught to most students. So I thought I would have a go at this one!
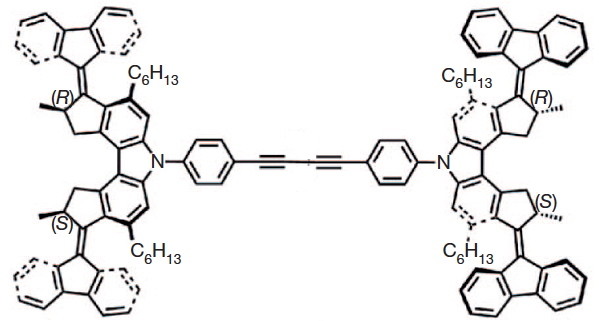
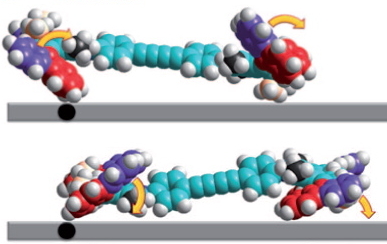
The car comprises a single (relatively small) molecule, shown above as the authors represented it. The motion along a surface comprised of copper atoms is driven by light as fuel coupled with encouragement from an STM probe. The distance travelled in a straight line was about 6nm in ten steps (note the nanodistance), although the average speed for the complete journey is not recorded. It is probably safe to say it was not recorded using a speed camera!
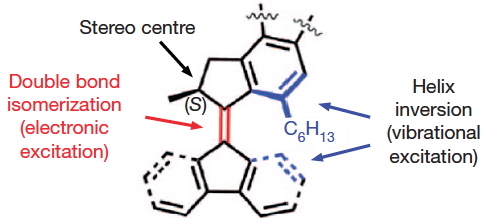
The chemistry is shown below. The car has four wheels (the fluorene units) which rotate about an C=C double bond axle using light as the fuel (a configurational change). The component labelled helix inversion can also be described by the chemical name atropisomerism, a topic I dealt with earlier with the example of Taxol and which is a conformational change.
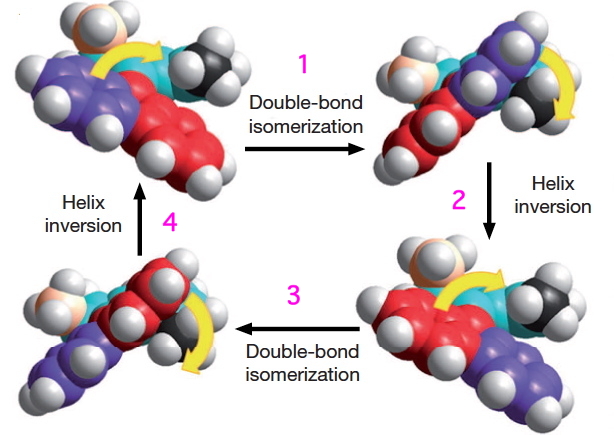
These two processes are used to rotate the wheels in the sequence shown below (after which the wheels return to their starting point).
I set out to build the car by optimising the 3D geometry of the molecule. This so that I could view the device from any direction (not just the one represented in the diagrams above). I also felt it important to estimate the change in energy of the car as the wheels rolled (something not touched upon in the original article). A good place to start would be to raid the supplementary information associated with the article. This comprises a PDF document and four movies. As it happens, none of these contain 3D coordinates for the molecule. Well, in truth this is not unusual, and I am used to such absence by now. Ah well, I would start from the top diagram, which is a schematic 2D representation of the molecule. As you can read in this post, such representations can often be illusory, or even contradictory. One is indeed lucky if they are free of ambiguity. Thus:
- The stereogenic centres are fine, they are labelled (R) and (S), and they provide an important aspect of the mechanism for allowing the motions of the four wheels to be coordinated such that the car drives in a straight line. Much is made of this aspect in the article.
- It is the atropisomerism that starts to cause problems. Here the diagram contains emboldened bonds carved into a benzene ring. This convention was first proposed by Hubert Maehr in 1985, but his intended use has since been much abused. As I fear it is here. Although it is difficult to be certain, the benzo groups in the car are annotated with several Maehr-like emboldened bonds, and a few non-Maehr wedged bonds as well. It is all meant to indicate perspective, and probably not intended in the Maehr sense at all.
- That latter feeling is reinforced when the benzo groups of the fluorene unit are annotated with dashed bonds replacing the single bonds in the Kekule resonance structure. Normally, a C- – -C is taken to indicate a breaking, or transition bond, but here it is again just an attempt at perspective (and a new addition to the bond menagerie).
Well, it is possible to build a 3D model armed with these instructions (although it has to be done visually, with constant comparisons with the space fill representations in the article).
- Here is my take on the starting point for the car:
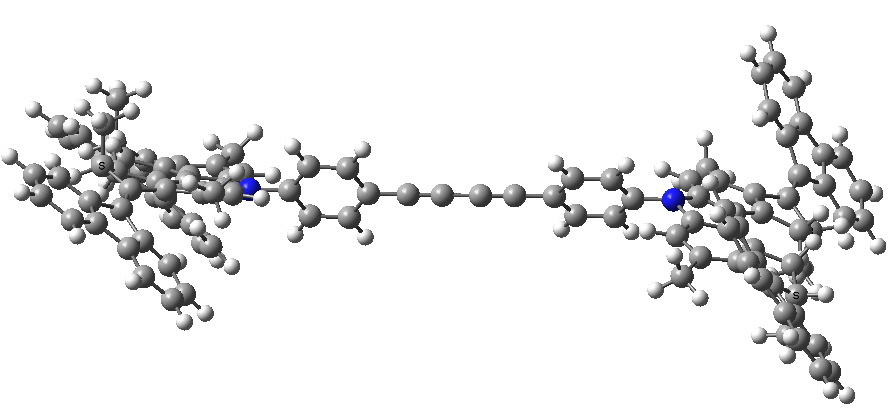
The initial conformation of the molecular car. Click for 3D.
- The car starts its journey by a light-driven rotation of the C=C bonds to form an isomer (about 8 kcal/mol higher according to my estimate using PM6).
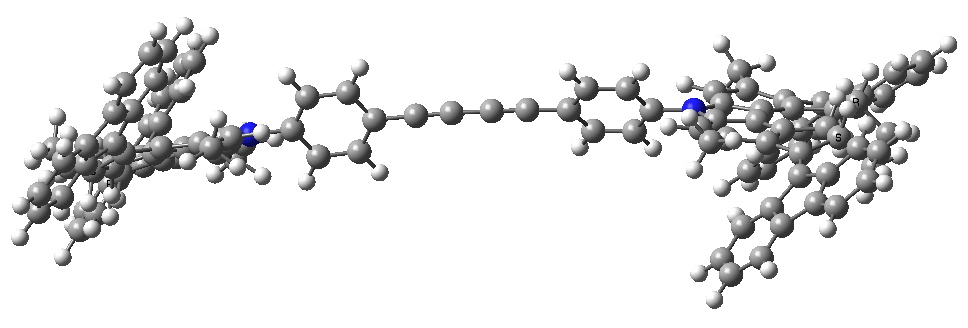
Car after step 1, double bond isomerisation. Click for 3D.
- There is then an STM-induced helix inversion, or atropisomerism. The two benzo groups are induced to swap over, much in the manner of bi-phenyls. The energy at this point is identical to the starting position. It is worth noting that the molecule was not returned to this position by reversing the first C=C rotation, but by two quite different operations (light and STM-electrons). I presume this was done to ensure the wheels turn in a constant direction, and do not simply flip back and forth randomly.
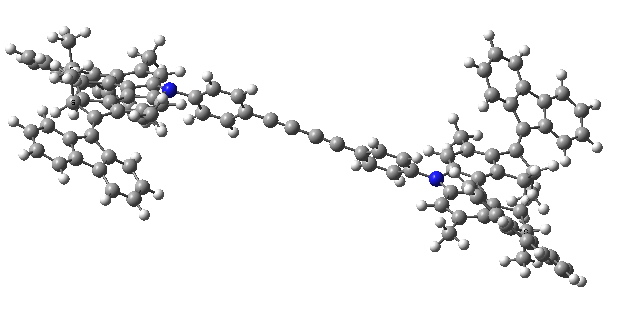
Car after step 2, helix inversion. Click for 3D.
- A final light-induced twist of the double bond (the energy is again about 8 kcal/mol higher than the start point)
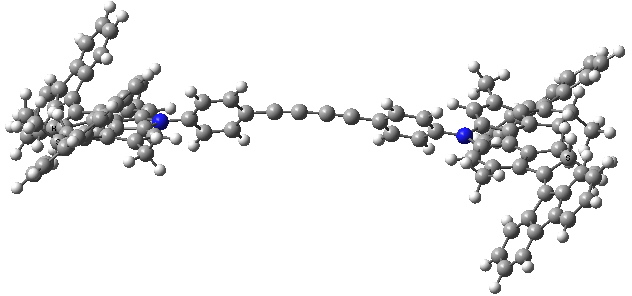
Car after step 3, double bond isomerism. Click for 3D.
- and another STM-induced helix inversion returns the car to ~0.6nm on from its starting position.
Postscript: The optimised ωB97XD/6-31G(d) geometries for the two poses of the car are to be found at 10042/to-10227 and 10042/to-10219 The total energy difference is 15.5 kcal/mol (compared with 8 at the PM6 level).
References
- T. Kudernac, N. Ruangsupapichat, M. Parschau, B. Maciá, N. Katsonis, S.R. Harutyunyan, K. Ernst, and B.L. Feringa, "Electrically driven directional motion of a four-wheeled molecule on a metal surface", Nature, vol. 479, pp. 208-211, 2011. https://doi.org/10.1038/nature10587
Tags: car drives, car rattling, chemical concepts, chemical name atropisomerism, chemical perspective, conformational analysis, day devices, energy, energy difference, Hubert Maehr, molecular car, nanocar, PDF, smallest car, Taxol
[…] world’s smallest nano car was recently driven a distance of 6nm along a copper track. When I saw this, I thought it might be interesting to go under the hood and try to explain what makes its engine […]
I remember reading this in Chemistry World.
Thanks for the extra explanations, I’ll be checking out the .fchk file in Gaussian if I have time and computing power.
Ben Feringa, the principle author of the article above, is one of the recipients of the 2016 Nobel prize for chemistry. See http://www.kva.se/en/pressroom/2016/the-nobel-prize-in-chemistry-2016/