A Matryoshka doll is better known as a Russian nesting doll. They can have up to eight layers. Molecules can only emulate two layers, although see here for a good candidate for making a three-layered example (the inside layer is C60, which itself might encapsulate a small molecule. See also DOI: 10.1021/ja991747w). These molecular dolls can be created out of quite simple molecules. Here I explore just one, and focus on what is happening inside!
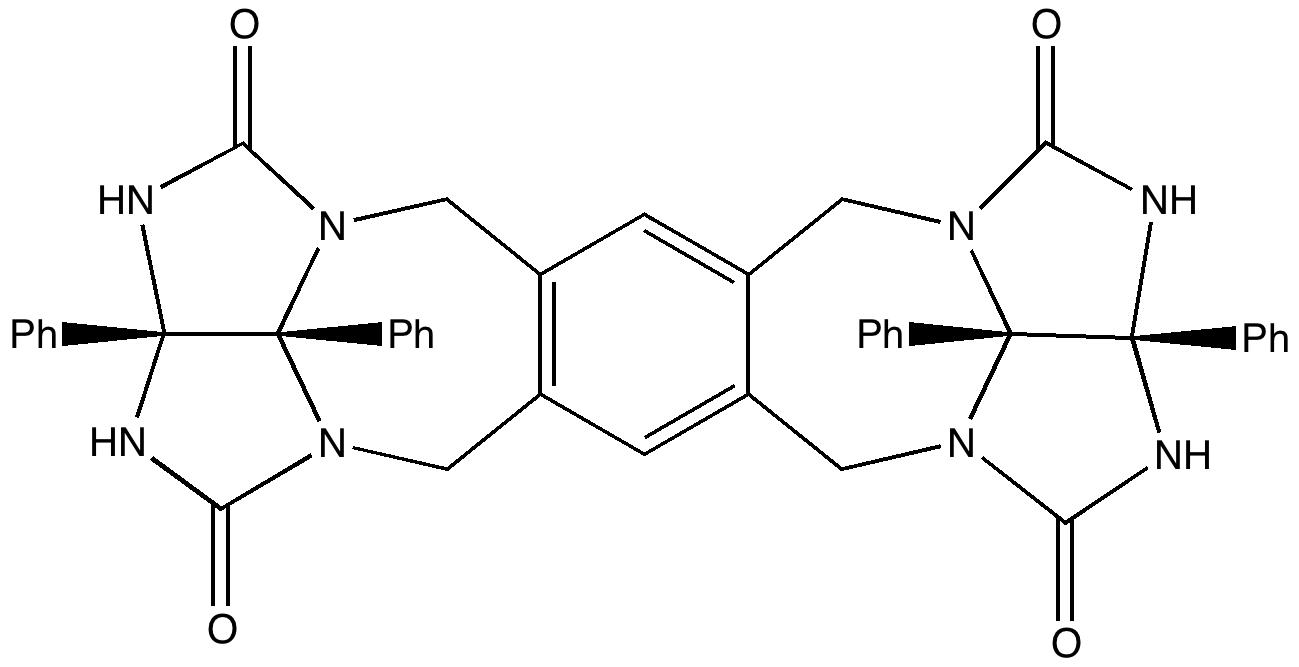
The above represents the “tennis ball” component of a molecule first made by Branda, Wyler and Rebek (DOI: 10.1126/science.8122107) in 1994. It has four pairs of carbonyl/NH units, and two of these molecules can stitch together to form an almost spherical capsule. Into this can pop smaller molecules, and in this case methane was persuaded to enter (highlighted with a magenta arrow below).
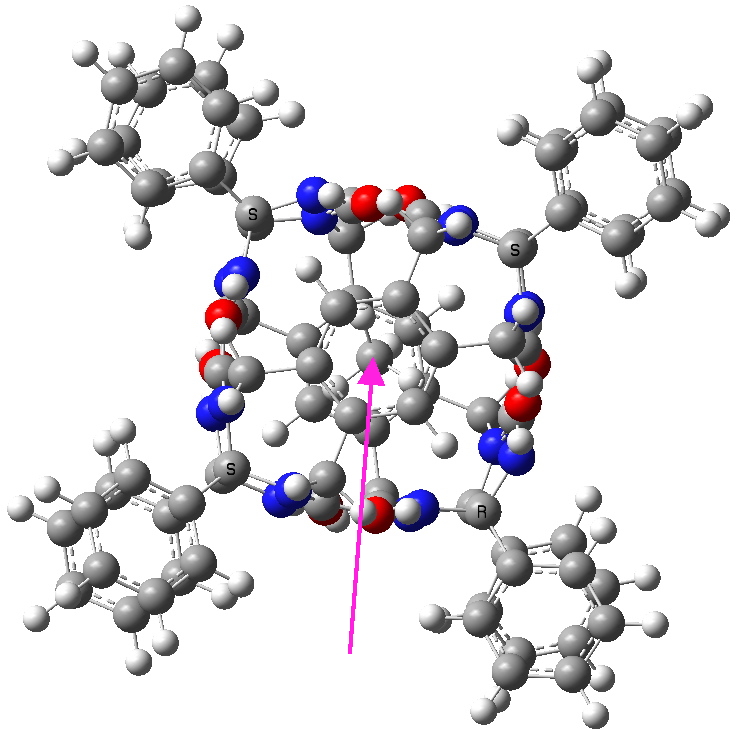
A molecular Russian doll with methane inside. Click for 3D
The above is a ωB97XD/6-311G(d,p)/SCRF=dichloromethane calculation (under optimum conditions, this can predict the shifts of protons to an accuracy of < 0.1 ppm!). So it is here, with the calculated methane chemical shift being -0.84 ppm (averaged over the four protons). In fact, the spectrum above is amazingly like the real thing (which can be seen at the DOI above), excepting of course proton couplings. Oh, if you cannot see a spectrum, it is because your browser does not support SVG. Why did I use this format? So that you can expand the view above (zoom in using your browser), and the SVG will rescale the drawing without loss of resolution!
We might presume then that the calculated structure must be a good model for the real thing (the structure of which Rebek and Co were never able to obtain). If you click on the model above, you may notice that the methane is not located exactly in the centre of the cavity, but it is displaced towards the face of one of the benzene rings, and away from the other. Thus these internal dolls do have a preference for where they sit, a phenomenon by the way which Rebek has termed social (molecular) isomerism (DOI: 10.1021/ja020607a).This system has 181 atoms. I estimate that this sort of calculation can readily be done for molecules with up to about 250 atoms nowadays, which would cover a fair sprinkling of these molecular Matryoshka dolls.
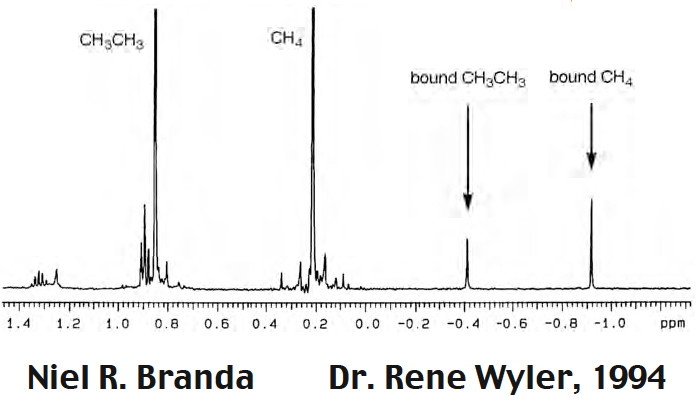
Postscript: Professor Rebek has kindly sent me the spectrum of both encapsulated methane and ethane which is reproduced below. The NMR of ethane calculated by the same procedure as above is -0.41 ppm.
Archived on 2011-09-26. URL:http://www.ch.imperial.ac.uk/rzepa/blog/?p=4930. Accessed: 2011-09-26. (Archived by WebCite® at http://www.webcitation.org/61zSZeG7P)