In 1923, Coster and von Hevesy[1] claimed discovery of the element Hafnium, atomic number 72 (latin Hafnia, meaning Copenhagen, where the authors worked) on the basis of six lines in its X-ray spectrum. The debate had long raged as to whether (undiscovered) element 72 belonged to the rare-earth group 3 of the periodic table below yttrium, or whether it should be placed in group 4 below zirconium. Establishing its chemical properties finally placed it in group 4. Why is this apparently arcane and obscure re-assignment historically significant? Because, in June 1922, in Göttingen, Niels Bohr had given a famous series of lectures now known as the Bohr Festspiele on the topic of his electron shell theory of the atom. Prior to giving these lectures he had submitted his collected thoughts in January 1922[2].
Like Mendeleev before, who had predicted ekasilicon, ekaaluminium and ekaboron (eventually discovered as germanium, gallium and scandium), Bohr had used his electron shell theory to (correctly) predict the properties of element 72. In modern terms, he had concluded that its electron shell structure must be 2.8.18.32.10.2 or [Xe].4f14.5d2.6s2. Classification as a rare earth would have resulted in the 4f shell having 15 electrons, impossible in Bohr’s theory. Coster and von Hevesy note in their article that Bohr’s striking prediction was now verified. Bohr latter told this story in his Nobel prize lecture.
Why I am writing all of this? For various reasons:
- Unlike Mendeleev, Bohr’s prediction of the properties of a (then uncharacterized) element, whilst famous at the time, is nowadays largely forgotten by chemists. It is one of the great achievements of the then new quantum theory.
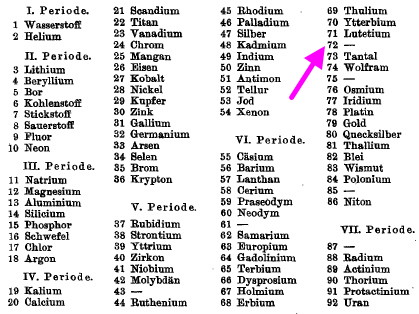
- Reading the 67 pages of Bohr’s article[2] on the topic reveals no discussion of element 72 (articles of this era are nowadays only available as scanned images, not full text, and one must rely on a human visual scan of all 67 pages, which of course may not be reliable) but its (absence) in the table below is striking. Here VI means the 6th row of the periodic table.
- Notice the only other missing elements, Technetium (43), Promethium (61), Astatine (85), Francium (87) and Rhenium (75, the only non-radioactive one remaining to be discovered),
- I must presume that Bohr introduced his discussion of element 72 into his June lectures to make an impact with his audience! One might have hoped that tracking down what happened between January 1922, when Bohr fails to make much of the missing element 72, and June in the same year would be possible from Coster and von Hevesy’s citation of Bohr in 1923. But it was the practice of the time to rarely cite one’s sources. Thus they give no published citation to Bohr, and one might conclude that they might instead be quoting Bohr from his lectures rather than his writings (poor old Bury, now forgotten![3]).
- Bohr’s own 1922 article on the topic is also visually striking. It contains in its 67 pages:
- 13 (short) equations
- Two figures (the second a variation on the first)
- One table (above).
- and lots of text (in German).
- No citations at the end, not even one, although many people are acknowledged in the text itself.
- No explicit statement of shell structures as e.g. 2.8.18.32.10.2 or [Xe].4f14.5d2.6s2.
Given that Bohr’s article can be regarded as one of the most influential of the 20th century (even prior to its being placed on a firm theoretical footing by solution of the Schroedinger equation for the hydrogen atom), I find it interesting how quickly it achieved this status (Bohr won the Nobel prize in 1922 as well). One might conclude that reputations were made as much via verbal presentations as by the immediate visual impact of the associated publications.
Finally, I note the striking contrast between Bohr’s article[2] and Langmuir’s[4], written about a year earlier in 1921, although first set out in 1919. Here, Langmuir[4] sets out some postulates, the first of which is shown below (and first explained in 1919)
The filled electron shells are clearly set out here (much more clearly than in Bohr’s 1922 article[2]). But yet again, we remain uncertain as to how Langmuir arrived at this postulate (perhaps here) Although he (very briefly) mentions Bohr in his own paper, it is only in the context of speculating about what prevents the electrons from falling into the nucleus, and few citations are again given (a notable exception is to Pease[5] for suggesting the triple bond). We may only suspect that Langmuir had heard Bohr talking about his theory, and had extended G. N. Lewis’ concept (also not directly cited) of (filled) valence shells for his own theory of chemical bonding.
Well, in a little less than 90 years, we have progressed from finding almost no sources cited in some of the most influential papers of the 20th century, to the DOI (or URL) embedded in everything. I think that when the history of the present era is written, the introduction of the DOI/URL will take its place in the pantheon of great scientific events. Its the connections that matter, stupid!
Postscript. Hevesy in this review[6] written in 1925 sets out a good history of Hafnium. This article contains (on p7) a clear statement of the electron shell structure of Hafnium as 2.8.18.32.8.2.2, which is cited as Bohr’s result. Hevesy quotes Bohr via reference 12, which is in fact to a book Bohr published in 1924. There is no mention of Langmuir in Hevesy’s review.
Postscript1: Hafnium (as its oxide) is now an essential element to the ever smaller fabrication of silicon chips (32nm and smaller). It is one of 14 elements considered essential to the future green technologies (six of which, but not including Hafnium, are considered in critical risk of supply disruption by 2015).
References
- D. COSTER, and G. HEVESY, "On the Missing Element of Atomic Number 72", Nature, vol. 111, pp. 79-79, 1923. http://dx.doi.org/10.1038/111079a0
- N. Bohr, "Der Bau der Atome und die physikalischen und chemischen Eigenschaften der Elemente", Zeitschrift f�r Physik, vol. 9, pp. 1-67, 1922. http://dx.doi.org/10.1007/BF01326955
- C.R. Bury, "LANGMUIR'S THEORY OF THE ARRANGEMENT OF ELECTRONS IN ATOMS AND MOLECULES.", Journal of the American Chemical Society, vol. 43, pp. 1602-1609, 1921. http://dx.doi.org/10.1021/ja01440a023
- I. Langmuir, "Types of Valence", Science, vol. 54, pp. 59-67, 1921. http://dx.doi.org/10.1126/science.54.1386.59
- R.N. Pease, "AN ANALYSIS OF MOLECULAR VOLUMES FROM THE POINT OF VIEW OF THE LEWIS-LANGMUIR THEORY OF MOLECULAR STRUCTURE.", Journal of the American Chemical Society, vol. 43, pp. 991-1004, 1921. http://dx.doi.org/10.1021/ja01438a003
- G. Hevesy, "The Discovery and Properties of Hafnium.", Chemical Reviews, vol. 2, pp. 1-41, 1925. http://dx.doi.org/10.1021/cr60005a001
Tags: Bohr, Bury, chemical bonding, Chemical IT, chemical properties, Copenhagen, General, green technologies, Hafnium, Historical, Langmuir, Niels Bohr, silicon chips, Technetium, X-ray


[…] theories in chemistry write a personal and anecdotal account of their work. Niels Bohr[1] was one such and four decades later Robert Woodward wrote “The conservation of orbital symmetry” […]
Since writing this post in 2011, I have found three more key references.
The first is an article by Bohr published in March 1921 in which the electron shells of the rare gases are clearly set out, and which forms the basis for much of the discussion above. doi 10.1038/107104a0
The second is one by the Bury (mentioned above as “chemical considerations”) and dated April 1921 (doi: 10.1021/ja01440a023) in which a detailed discussion of the electronic shells of the entire periodic table is undertaken, far more than was attempted a month earlier by Bohr. Bury very clearly writes “between lutecium and tantalum an element of atomic number 72 is to be expected. This would have the structure (2,8,18,32,8,4) and would resemble zirconium“. It was of course the about to be discovered Hafnium. This appears to be the very first, Markovnikov-like, prediction of the expected properties of this element. Bury also mentions element 75 (also undiscovered at the time, but now known as Rhenium).
Bury in turn references an earlier article in 1919 by Langmuir (doi: 10.1021/ja02227a002) where the numbers 2,8 and 18 first appear. However this latter article, long and detailed as it is, does not set out the shell structures of the atoms as Bury does. The message there is not clear! If you compare this 1919 article with the one Bury published in 1921, the latter looks almost completely modern, the former most certainly does not. Nevertheless by July 1921, Langmuir (with the help of Bohr and Bury, but not perhaps clearly acknowledged by him as such) had clearly tidied his act up (doi: 10.1126/science.54.1386.59), although element 72 is not discussed there.
Given the fame that accrued to Markovnikov for his own predictions of undiscovered elements, it is tempting to conclude that more credit should be given to Bury in 1921 for his own prediction of Hafnium and his almost completely modern analysis of the electronic structure of the periodic table.
2019: The year celebrating the Periodic Table.
As part of my contribution, can I highlght this amazing collection of Periodic tables; https://www.meta-synthesis.com/webbook/35_pt/pt_database.php which i Marl Leach’s collection of examples.
From the time of Mendeleev’s periodic table, chemist had begun to notice groupings of elements in 2 and especially 8. In 1904 Richard Abegg was one of the first to extend the concept of coordination number to a concept of valence in which he distinguished atoms as electron donors or acceptors, leading to positive and negative valence states that greatly resemble the modern concept of oxidation states. Abegg noted that the difference between the maximum positive and negative valences of an element under his model is frequently eight. The rule of 8 or octet rule was called Abegg’s rule in 1904. This was appropriated by Langmuir in 1919 and later by Bohr who took the credit themselves or at least did not object to others crediting them. See Wikipedia “octet rule” for references. Similarly Bohr took the formula created by Balmer and enlarged by Rydberg for his atomic model fully intact. He just added it to the formula for astronomical calculation of orbits adding Planck’s constant. The book Einstein and the Quantum credits Bohr for predicting the Hydrogen Lyman series, but this had been predicted 20 years earlier by these pre-existing formulas. References in Wikipedia under Balmer formula and Rydberg formula.
Thanks for all these interesting historical notes.
I am adding a DOI for Abegg’s article if anyone wishes to read it: DOI: 10.1002/zaac.19040390125.