Observation of the slow racemization of isobornyl chloride in a polar solvent in 1923-24 by Meerwein led to the recognition that mechanistic interpretation is the key to understanding chemical reactivity. The hypothesis of ion pairs in which a chloride anion is partnered by a carbocation long ago entered the standard textbooks (see DOI 10.1021/ed800058c and 10.1021/jo100920e for background reading). But the intimate secrets of such ion-pairs are still perhaps not fully recognised. Here, to tease some of them them out, I use the NCI method, which has been the subject of several recent posts.
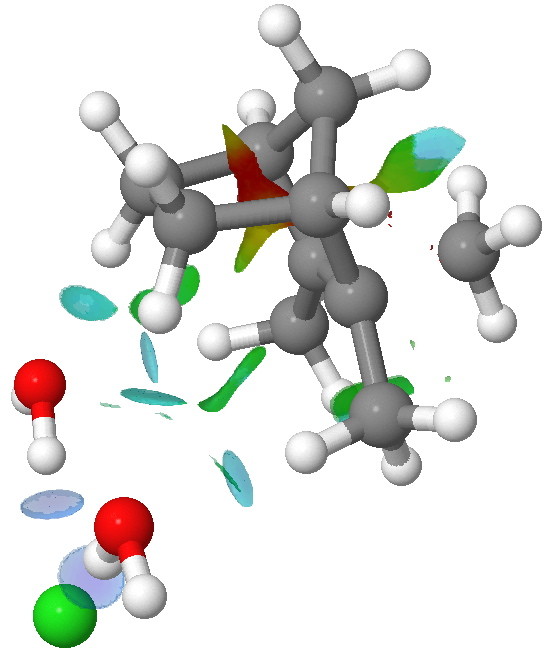
NCI analysis of the iod-pair transition state for methyl migration in isobornyl chloride. Click for 3D.
To remind, the colour coding of the NCI surface is
blue=strongly attractive,
red=strongly repulsive,
green=weakly attractive,
yellow=weakly repulsive. Shown above is the
ion-pair transition state for [1,2]methyl migration. Note how the hydrogen bonds between the chloride anion and the water molecules are clearly blue. Only slightly weaker (with a turquoise tint) is a pair of hydrogen bonds between the oxygen atoms and H-C bonds in the isobornyl cation. Such C-H…O bonding in ion-pairs seems to be particularly important. There are other blue regions, between an H…H pair, and a C-H bond and the carbon of the migrating methyl group. Also noteworthy is that many atom pairs have multi-coloured NCI regions, suggesting the interaction is not homogenous, and can be both attractive AND repulsive between any pair of atoms.
The NCI plot below shows the competing 1,6-hydride shift in isobornyl chloride, again involving an ion-pair transition state.
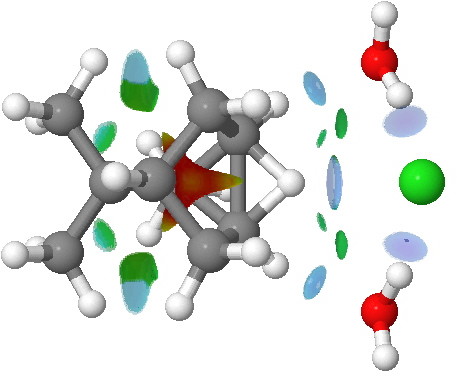
NCI surfaces for the 1,6 hydride migration transition state in isobornyl chloride. Click for 3D.
Notice in this example how the migrating hydrogen supports an attractive hydrogen bond to the chloride anion (ostensibly between a hydride atom and an anionic chloride?), and again how there are a number of
blue regions elsewhere.
Modelling is increasingly focusing on these weaker interactions, that probably mediate much (stereo)selectivity in organic reactions. How long before such approaches themselves enter the text-books?
-
Henry Rzepa is Emeritus Professor of Computational Chemistry at Imperial College London.
View all posts
Related
Tags: chemical reactivity, ion pair, isobornyl, Julia Contreras-Garcia, nnon-covalent-interactions, watoc11
This entry was posted on Sunday, May 29th, 2011 at 9:11 am and is filed under Interesting chemistry. You can follow any responses to this entry through the RSS 2.0 feed.
You can leave a response, or trackback from your own site.

