The two previous posts have explored one of the oldest bonding rules (pre-dating quantum mechanics), which postulated that filled valence shells in atoms forming molecules follow the magic numbers 2, 8, 18 and 32. Of the 59,025,533 molecules documented at the instant I write this post, only one example is claimed for the 32-electron class. Here I suggest another, Nobelocene (one which given the radioactive instability of nobelium, is unlikely to be ever confirmed experimentally!)
Nobelium has the electronic configuration [Rn].5f14.7s2 , which means the 6d and 7p shells are still empty. Filling these would take 10+6 electrons, or four more electrons (20) if one starts from No4+, resulting in a complete 32-electron filled shell for the Nobelium. These twenty electrons could be provided by two cyclo-octatetraenyl (COT) dianion ligands. Nobelium, with its nuclear charge of +102, has highly relativistic inner-shell electrons, and so special techniques must be used to model this. Here I have used a SARC all electron relativistically contracted basis set (DOI: 10.1021/ct100736b), to be used with the Douglas−Kroll−Hess scalar relativistic Hamiltonian (for details, see here). The QTAIM analysis is shown below (quite a spider’s web):
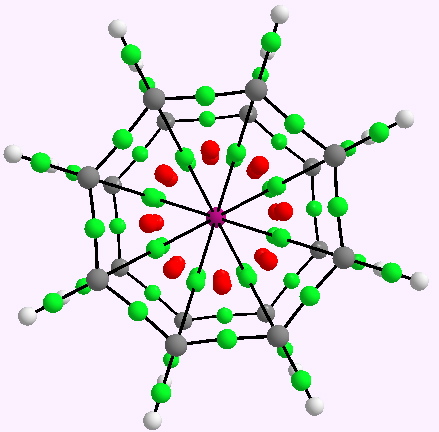
Nobelocene. AIM analysis. Click for 3D.
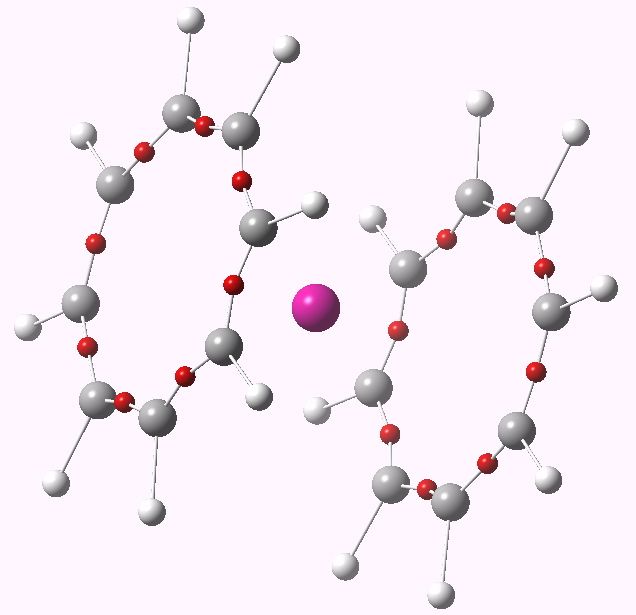
ELF analysis for Nobelocene.
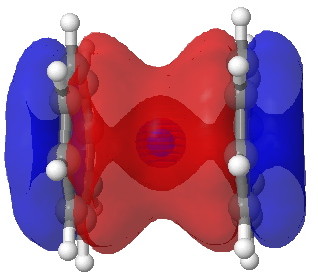
Molecular orbital for nobelocene. Click for 3D.
Hi Henry 🙂
Saw your Nobelocene entry. I was wondering why Jmol displays pink small atoms: they are labeled Bq, so I thought “becquerel”, but there’s no such element. That’s why Jmol doesn’t recognize it.
What is the Bq meant for? Maybe Bk?
These are one of two designations used to indicate a dummy or other coordinate centre which has no assigned electrons or charge. X is used for coordinate definitions (as perhaps bond or ring centroids) and Bq for (in this example) the centroid of an ELF basin. If you took a close look at the coordinates, you might also have spotted that they are actually in Bohr, not Å. This I use to prevent some algorithms which are very fond of automatically connecting bonded pairs based purely on distance. One does not really want multiple bonds to Bq atoms, so using Bohr normally inhibits that automatic connecting process! Hope this clears the mystery up Angel!
Dear Henry,
well, to call a bond a bond or an interaction according to its strength is a question of gusto, or even arbitrary. Anyway, did you perform a vibrational analysis? If all wavenumbers are real, then it is a (meta)stable (bonded) molecule…
Best regards, Rene
I see 32 valence electrons for B32H32 and 18 for ferrocene. How many valence electrons do you count for uranocene and does it increase linearly for the actinides cenes between U and No? Also, Yb is above No in the periodic table. So, wouldn’t you also predict YbC16H16 is a magic number molecule? Yb appears to me to be more practical that No. So, has it been made?