In discussing ferrocene in the previous post, I mentioned Irving Langmuir’s 1921 postulate that filled valence shells in what he called complete molecules would have magic numbers of 2, 8, 18 or 32 electrons (deriving from the sum of terms in 2[1+3+5+7]). The first two dominate organic chemistry of course, whilst the third is illustrated by the transition series, ferrocene being an example of such. The fourth case is very much rarer, only one example ever having been suggested[1], it deriving from the actinides. In this post, I thought I would augment ferrocene (an 18-electron example) with beryllocene (an 8-electron example) and then speculate about 32-electron metallocenes.
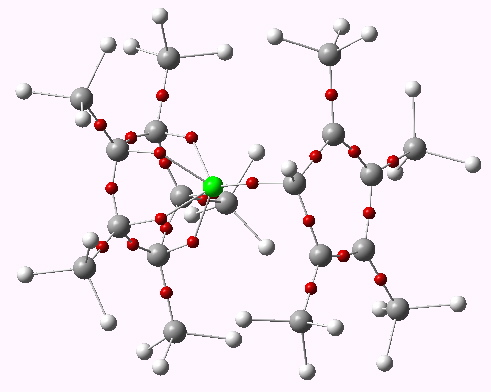
Cp*-beryllocene. ELF analysis. Click for 3D.
Uranocene is a rather different beast. The ligands are not cyclopentadienyl, but cyclo-octatetraenyl. Uranium has a radon core, and a 5f3, 6d1 and 7s2 valence shell(s) electron configuration. Ionised to U4+, formally the 5f, 6d and 7p shells are all empty; a total of 14 + 10 + 6 electrons would be required to achieve a 32-electron filled shell , or 30 additional electrons. The two COT ligands, as di-anions (achieving aromaticity) could provide only 20. So uranocene (Cambridge refcode URACEN10, DOI 10.1021/ic50111a034) is far from the holy-grail of a 32-electron complete molecule.
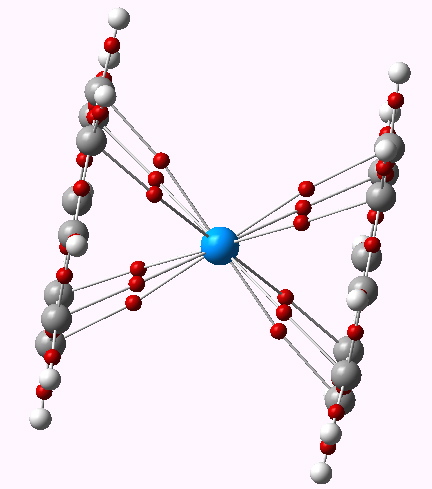
Uranocene. AIM analysis. Click for 3D
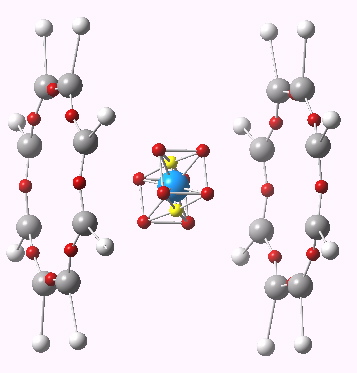
Uranocene. ELF analysis. Click for 3D
References
- J. Dognon, C. Clavaguéra, and P. Pyykkö, "Towards a 32‐Electron Principle: Pu@Pb<sub>12</sub> and Related Systems", Angewandte Chemie International Edition, vol. 46, pp. 1427-1430, 2007. https://doi.org/10.1002/anie.200604198
- M.M. Conejo, R. Fernández, D. del Río, E. Carmona, A. Monge, and C. Ruiz, "Synthesis and structural characterization of Be(η<sup>5</sup>-C<sub>5</sub>Me<sub>5</sub>)(η<sup>1</sup>-C<sub>5</sub>Me<sub>4</sub>H). Evidence for ring-inversion leading to Be(η<sup>5</sup>-C<sub>5</sub>Me<sub>4</sub>H)(η<sup>1</sup>-C<sub>5</sub>Me<sub>5</sub>)", Chem. Commun., pp. 2916-2917, 2002. https://doi.org/10.1039/b208972f
Tags: 8-electron rule, beryllocene, Cambridge, Irving Langmuir, metal, pence, uranocene
Incidentally, we’ve had good results using the SARC basis sets for topological analysis of actinide compounds rather than than the ANO-RCC basis sets or pseudopotentials – they’re segmented and of a fairly reasonable size, so you can use them pretty speedily in Gaussian.
[…] Henry Rzepa Chemistry with a twist « Beryllocene and Uranocene: The 8, 18 and 32-electron rules. […]
Thanks Ian. I have incorporated your suggestion of a SARC basis into the next post.
The interesting feature of berylocene is its extreme fluxionality (see JACS 1978, 100, 5695 for my very early paper with Streitwieser and Schaefer). As I recall, the ionic magnesocene prefers a symmetrical sandwich structure.
Yes, as systems with a lot of ionic character, they can easily adopt different coordinations. Thus beryllocene I believe has a (calculated) D5 symmetric structure which in fact is not a lot higher in energy than the asymmetric one noted here.
Magnesocene has its own version of an 8-electron rule. With each cyclopentadienyl anion moiety contributing six electrons it looks like a covalent structure would have 12 electrons around magnesium (the compound is strongly but not completely ionic). But if you look at orbital symmetries you find that two of the occupied cyclopentadienyl ring orbitals have no overlap with Mg and are essentially nonbonding to the bridge. Thus Mg has only eight valence electrons after all.
Beryllocene, however, cannot hold this sandwich structure. The bridge-“nonbonding” ring orbitals described above become significantly antibonding within the bridge if they are brought too close together, and Be unlike Mg is too small to prevent this. Beryllocene therefore is prone to have a ring slip out of the sandwich ro relieve this antibonding interaction … even at the cost of aromaticity in the slipped ring!
Dear Dr. Rzepa,
Thank you very much for the fascinating blog post regarding the 32-electron rule. Isn’t LuCp3 32 electron metalllocene complex? https://pubs.acs.org/doi/10.1021/om100240m
Thank you,
Satoshi Takebayashi
Thank you Satoshi for that suggestion. Lutetium has the shell structure
[Xe].4f14.5d1.6s2 which when ionised to the 3+ system becomes formally
[Xe].4f14. or in full
1s2.2s2.2p6.3s2.3p6.3d10.4s2.4p6.4d10.5s2.5p6.14f.5d0.6s0. (where 14f is added by the lanthanoids and [Xe] is shown in bold).
Summing the shells gives: 2.8.18.32.8.
Adding three Cp– units adds 18 electrons to the valence shell, hence yes formally
[Xe].4f14.5d10.6s2.6p6 or again in full
1s2.2s2.2p6.3s2.3p6.3d10.4s2.4p6.4d10.5s2.5p6.14f.5d10.6s2.6p6
and shells sum: 2.8.18.32.18.8
I note that Lu(Cp*)3 is a known crystal structure (DOI: https://doi.org/10.1021/om050709k) and that further coordination to this motif by thf is also known, ie https://doi.org/10.1016/S0020-1693(00)84558-2
Dear Dr. Rzepa,
Thank you very much for your extensive explanation and calculation. I have a foolish question. What does 4f32 mean? I thought 4f orbital only takes up to 14 electrons…
It seems like we can not simply count valence electrons like organometallic chemists do for the d-block complexes (18-electron rule). Is it because electrons in f orbitals behave differently? For example, I read 4f orbital is too compact to be considered for valence electron counts. I have no idea if it is true. (https://pubs.rsc.org/en/content/articlelanding/2012/sc/c2sc20448g)
Is there a simple way to say if a complex fulfills 32-electron criteria or not?
Satoshi, You are of course correct, and 14f is the lanthanoid contribution, not 4f32, which was an incorrect juxtaposition of total shell and f-shell. To make this clear, I have expanded the shell to show all the shells and not abbreviation to eg [Xe].
Dr. Rzepa, thank you for the clarification. So, LuCp3 is formally 32-electron. It is peculiar that THF coordinates to this complex which will form a formal 34-electron complex. It seems like 32-electron rule (if exists) does not apply to this complex. Maybe it is something to do with a filled 4f orbital. 3d10 Zn(II) complexes also forms formal 20-electron complexes.
I’m wondering if it is worth trying to synthesize 32-electron Th or U metallocene.
Thank you for sharing your thinking.
Satoshi