In an earlier post on this topic,[1]‡ I described how the curly-arrows describing the mechanism of a nucleophilic addition at a carbonyl group choreograph in two distinct ways, as seen in red or blue below. The arrows in red can be described as firstly addition to the carbonyl group to form either a transient intermediate (a two-step process) or instead a formal transition state state as a concerted single-step mechanism. The blue arrows do the reverse; firstly elimination and then followed by addition. I will use the shorthand AE for the first type and EA for the second type. Here I explore some more nucleophiles to see which of these two mechanisms they follow. Data for these results can be found at 10.14469/hpc/13171
N- carbon ylid: This is a very facile (low-barrier) reaction with a C-O bond length response that initially increases steeply, followed by a more modest decline and hence corresponds to an AE mechanism.
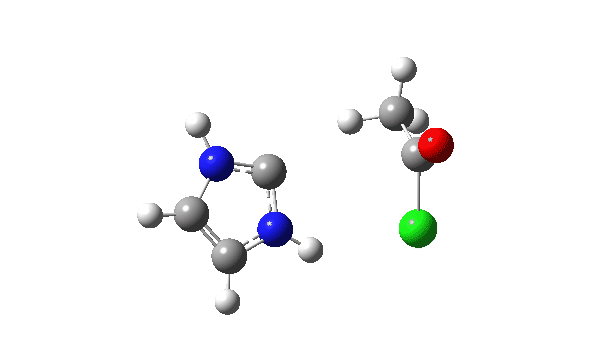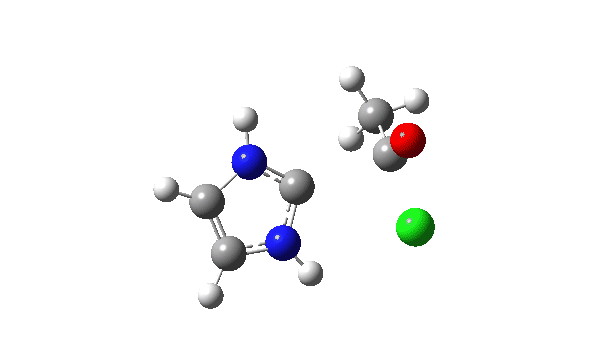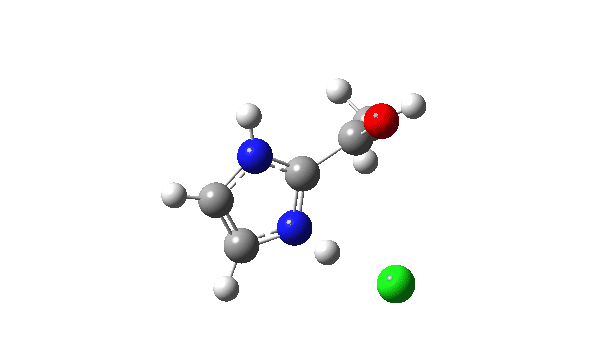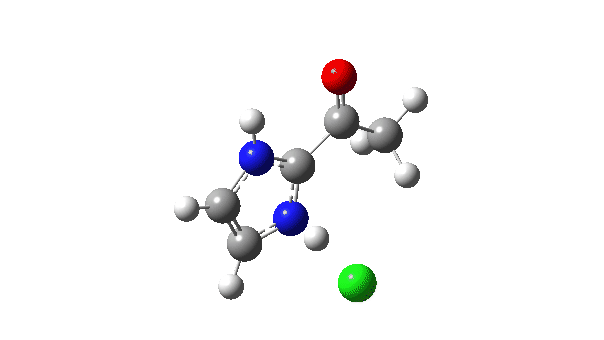
P carbon-Ylid: Essentially identical to the previous example, and again an AE mechanism.
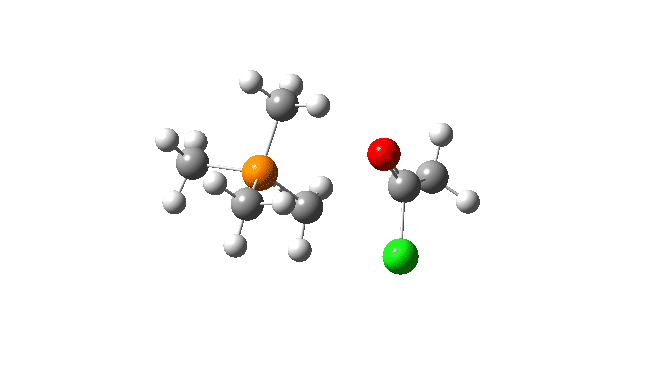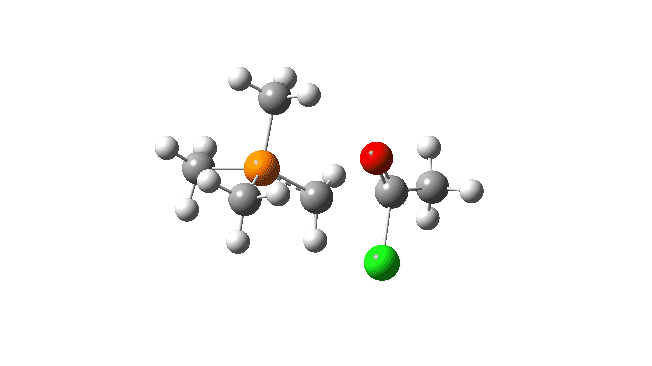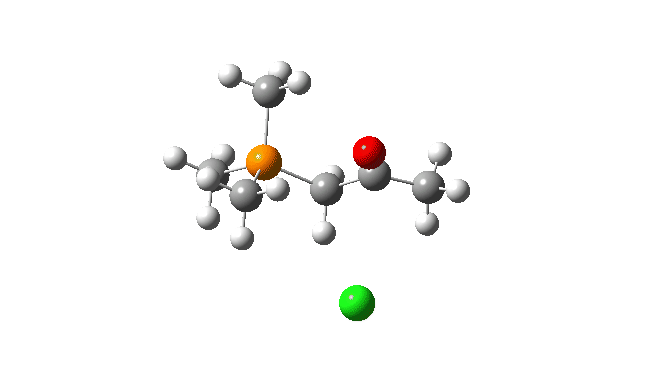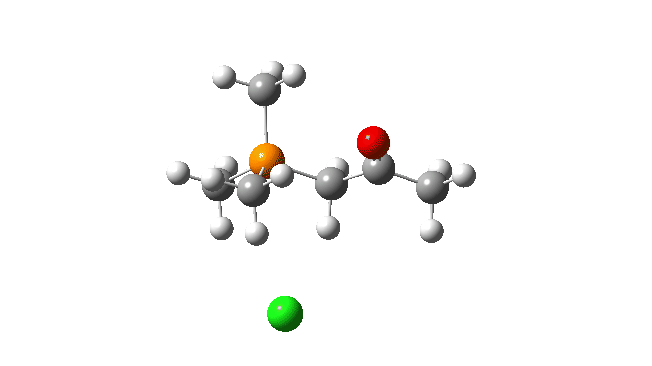
S carbon-ylid: Again, an AE mechanism.
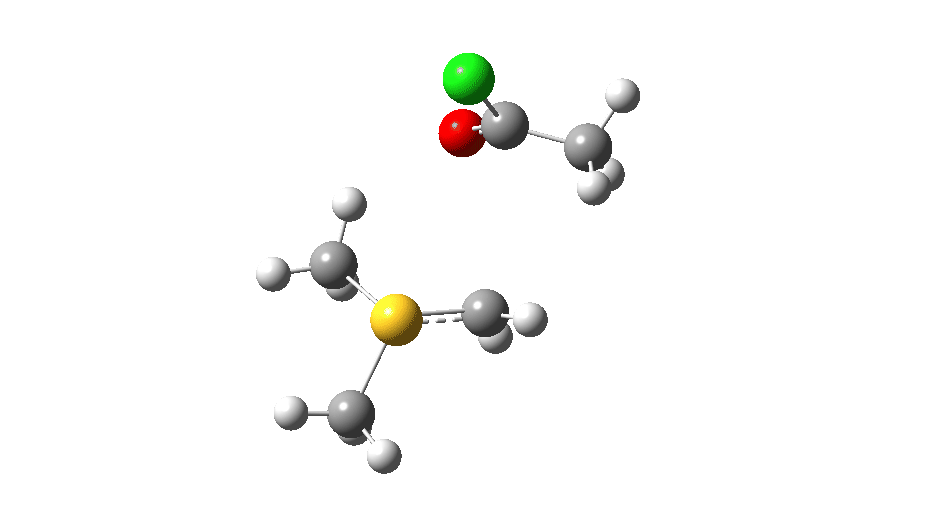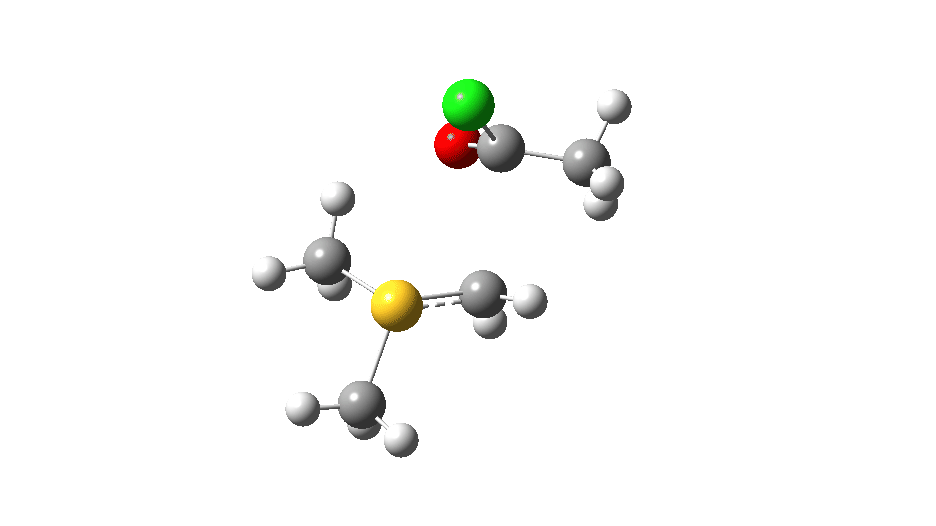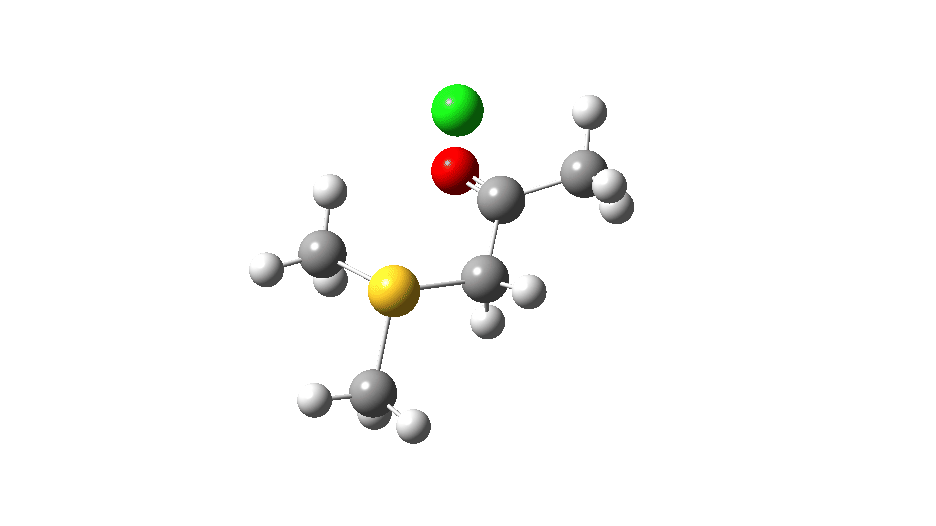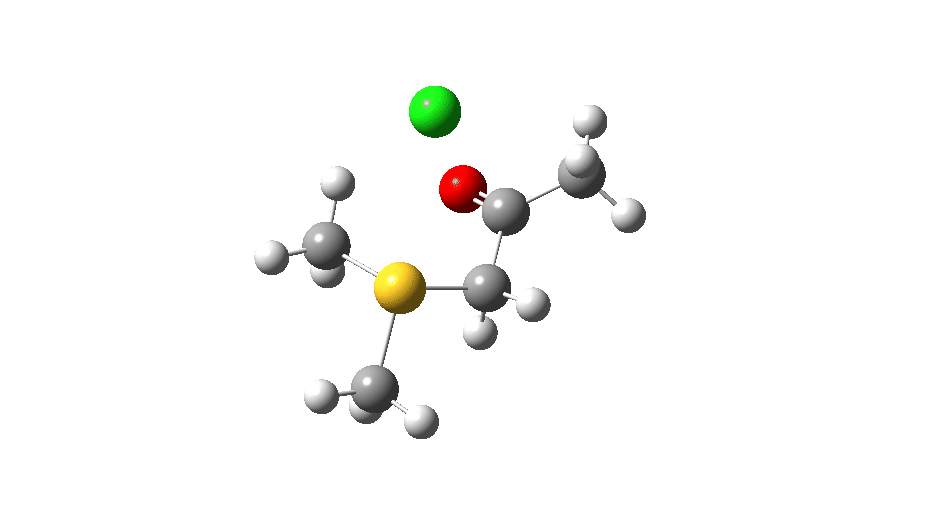
S-nucleophile: This one is different, showing a larger barrier and initial small decrease in the C-O length followed by a larger increase. This one is an EA mechanism.
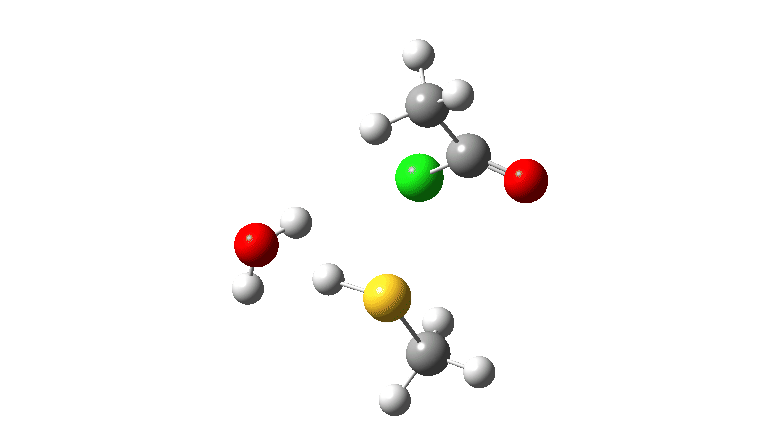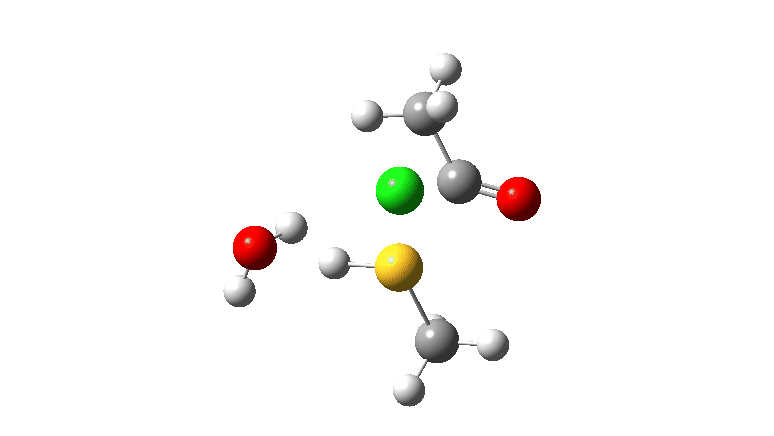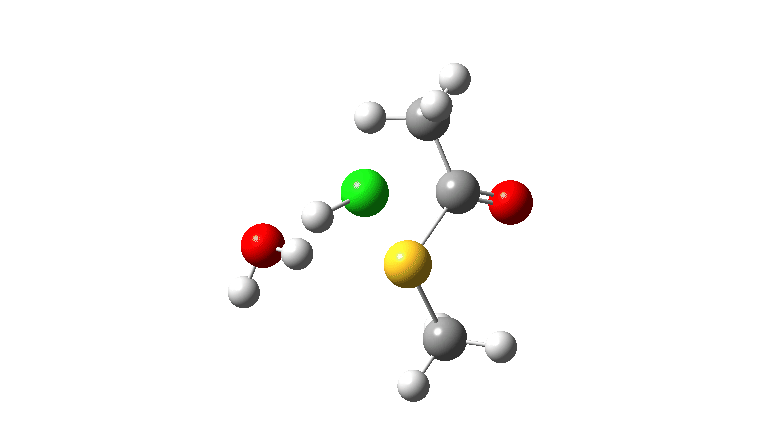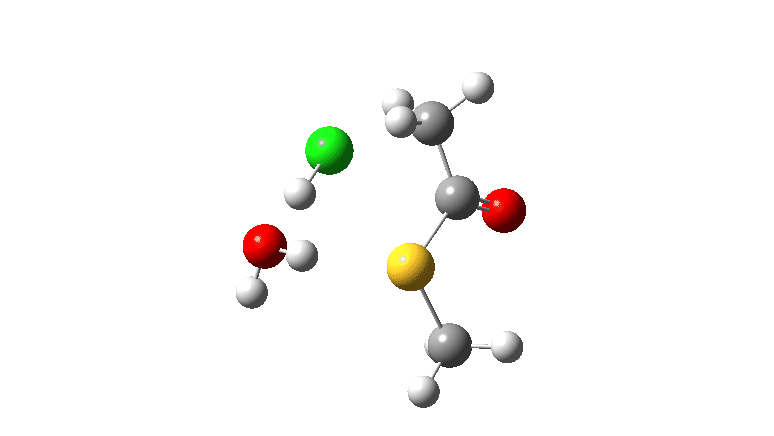
As I noted previously, it would be useful to have two double headed curly arrows available in palletes of these; <—> (AE) and >—< (EA) to illustrate the difference between the two mechanistic types.
‡This is the first instance where I cite a blog using a CrossRef DOI generated for it. Previous such citations used a DataCite DOI, which the bibliographic software used here to add them to the post (Kcite) does not support.
References
- H. Rzepa, "The "double-headed" curly arrow as used in mechanistic representations.", 2023. https://doi.org/10.59350/f00wf-5tq46
Tags: Interesting chemistry