The schematic representation of a chemical reaction mechanism is often drawn using a palette of arrows connecting or annotating the various molecular structures involved. These can be selected from a chemical arrows palette, taken for this purpose from the commonly used structure drawing program Chemdraw. Explanations of how to apply the individual arrows are not always easy to find however! Circled in red are the ones to be discussed here, although most carry fascinating and often subtle meanings!‡
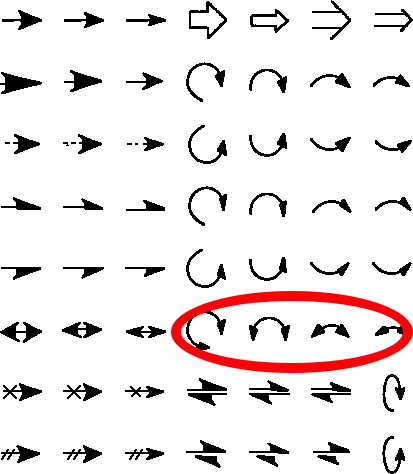
The most common meaning of the double-headed arrow is probably best illustrated by the scheme below, which involves the addition of a nucleophile to a carbonyl compound, forming a presumed “tetrahedral” intermediate, which is then immediately followed by the eviction of a leaving group – the chloride anion in the example below. The two red arrows show an electron pair firstly moving to the oxygen, and then with the reverse arrow 2 back to reform the carbonyl group. This process is called an addition/elimination mechanism. It is therefore tempting to conflate the two steps into one by instead using a double-headed arrow (3, blue), which if nothing else, saves a little bit of time in the drawing – a useful examination technique!
Of course, the top scheme (red arrows) is a two-step process, involving a discrete (tetrahedral) intermediate and two transition states. The conflated scheme below it (blue arrows) might imply (or not) a single-step process with a single transition state. Since few people who draw such schemes have any information on whether it is a two-step or a single step process, the actual chemical meaning of the double-headed arrow is left implicitly ambiguous, without implying anything about how many discrete steps are involved. However, it is tempting to conclude that the first red arrow (1) reduces the double bond order of the carbonyl group to a single bond, which might therefore be expected to lengthen and the second red arrow (2) reforms the double bond, thus shortening the bond. The two arrows clearly do not move simultaneously. The conflated third arrow (3) leaves the status of the carbonyl bond length changes undefined, or might it mean that it first gets longer and then shorter along the reaction path, depending of course on which moves first!
Enter computation, where the energy pathway of such a reaction can be computed, along with geometries at each stage. Here I explore three examples† to see what results (ωB97XD/De2-TZVPP/SCRF=DCM), FAIR DOI: 10.14469/hpc/13171
Acetyl chloride + Methanol.
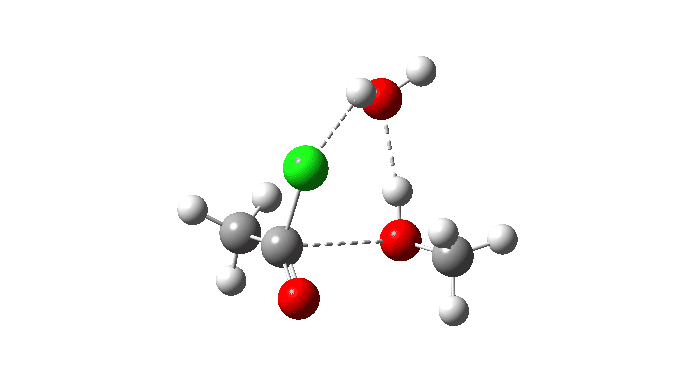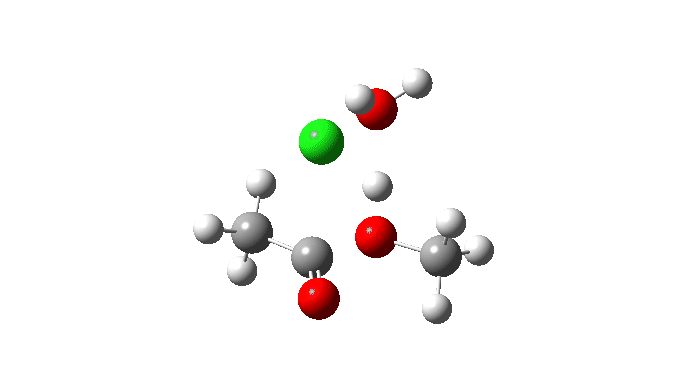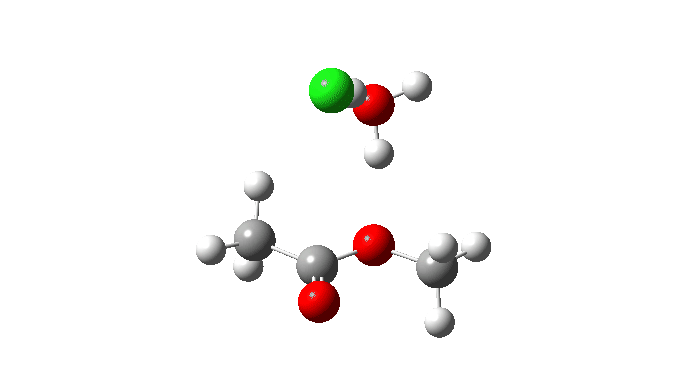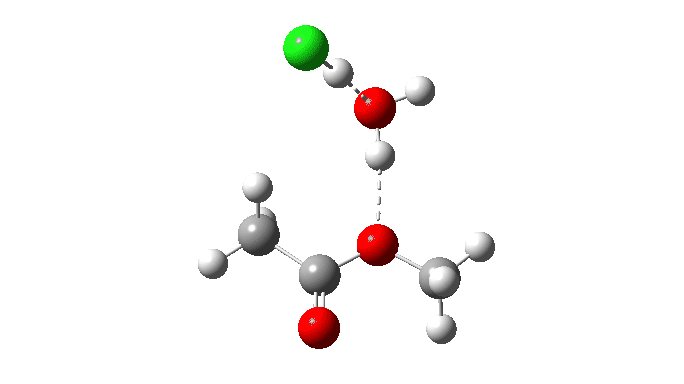
This uses a model in which a proton transfer from the methanol to the chloride anion is facilitated by water. This enables (but does not enforce) a continuous concerted process to occur. This emerges from the computed intrinsic reaction coordinate (IRC) as having a low barrier and an exothermic reaction, which agrees with experimental observation. The required proton transfer is part of the concerted process, albeit occurring in a second lower energy stage (IRC ~+1.5).
But take a look at how the carbonyl bond length changes along this IRC. It first shortens, and only starts to lengthen as the chloride is evicted. So the carbonyl group actually contracts in length at the transition state, the opposite of what might be inferred by using a double-headed arrow.
Also included is the dipole moment response, which does seem to correspond to the formation of an ionic intermediate!
Acetyl chloride + HF.
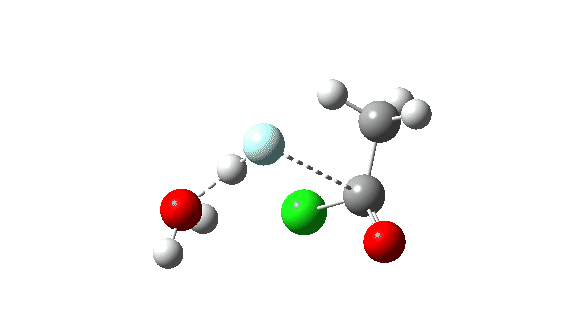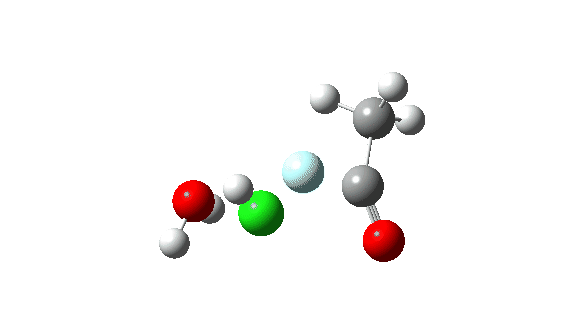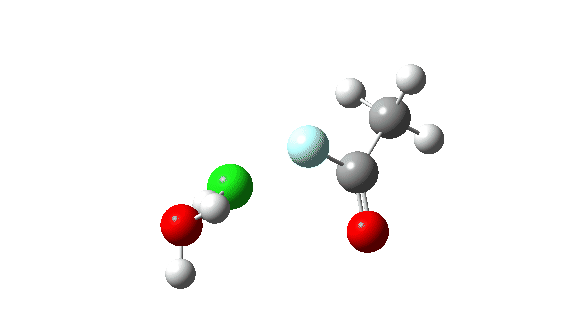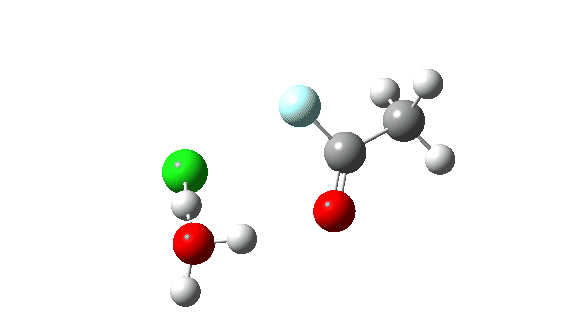
Hydrogen fluoride as a nucleophile replacing methanol shows a much higher barrier, since it is less good as a nucleophile in this context.
Again, observe the bond length response of the carbonyl group, which is at its shortest at the (single step) transition state.
This corresponds to a different interpretation of the double-headed arrow, as per below, but occuring as part of a single concerted process not involving any intermediate.
The dipole moment response is rather different from methanol.
Acetyl chloride + Methylamine.
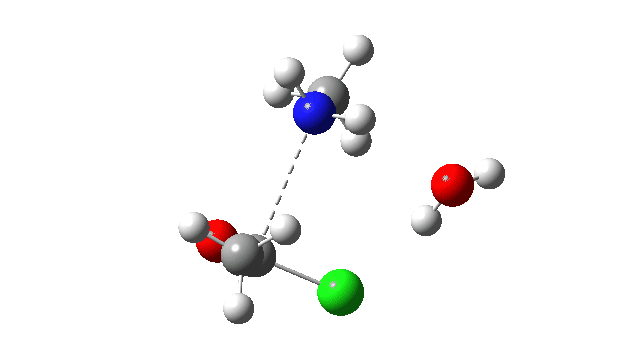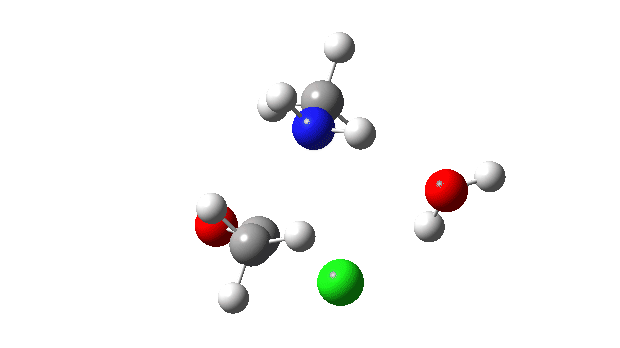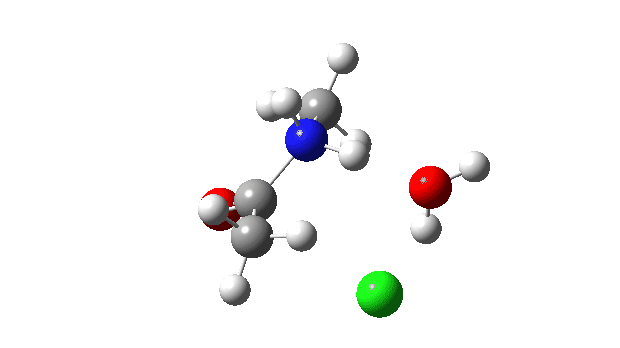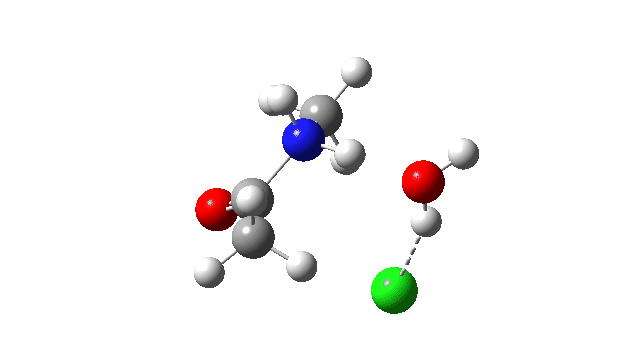
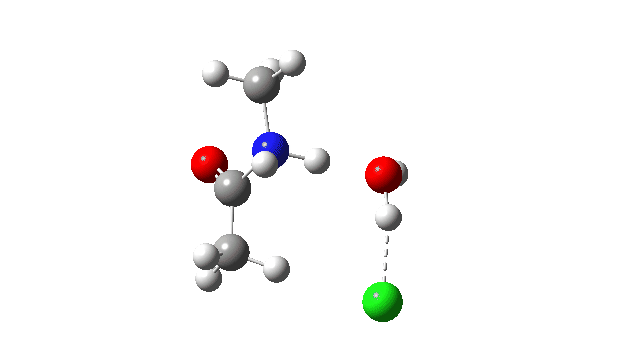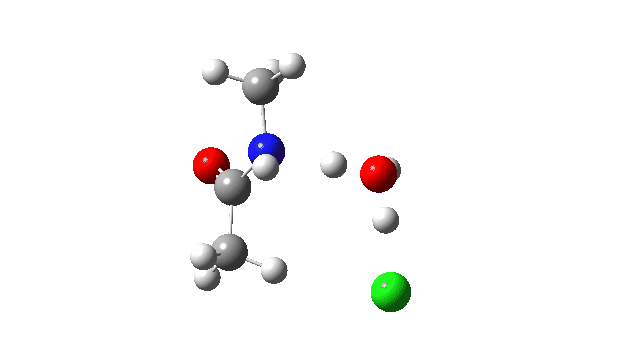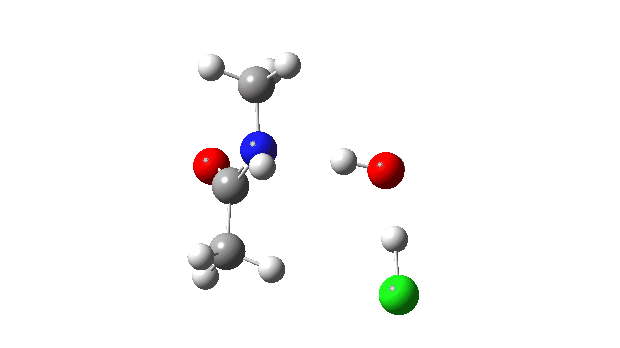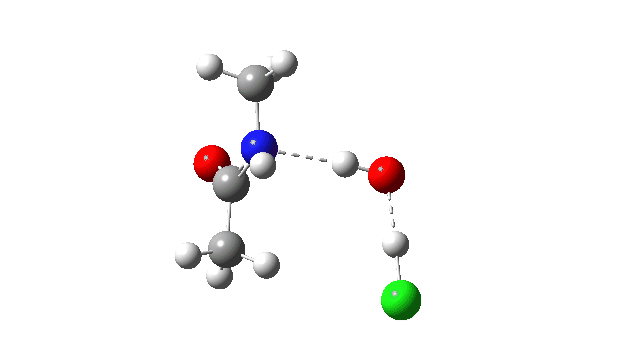
The energy profile now shows two distinct transition states (IRC ~7 and again at 0.0). The first is a very low energy addition to the carbonyl group with concerted eviction of the chloride anion, which only hydrogen bonds to the water shown. The second stage is the proton transfer from the nitrogen to the water and thence relayed to the chloride anion, for which a transition state at IRC ~0.0 is found.
But now observe the bond length response, which shows a distinct maximum around the first transition state (IRC ~7). This is the opposite behaviour to the previous two systems, and now indeed matches the original inferences one might make from the double headed arrow.
So we can conclude that there are in fact TWO types of double-headed arrow which could be used in mechanistic representations. The first is when arrow 1 is ahead of arrow 2 (red), resulting in initial weakening of the carbonyl bond. The second is when arrow 4 is ahead of arrow 5, resulting in initial strengthening of the carbonyl bond.
Perhaps to avoid confusion, we really need two different representations of a double-headed arrow to clearly differentiate them! Perhaps a reversal of the direction of the arrowhead? But that does not (yet?) exist in the Chemdraw palette.

‡This is part of the arcane “knowledge” of chemistry which is often absorbed rather than learnt by students of the subject, but which as a result becomes a language that becomes inscrutable to anyone else! †Another example was noted in the previous post.
Tags: Curly arrows