One of the important aspects of chemical reaction mechanisms is the order in which things happen. More specifically, the order in which bonds make or break when there are more than two involved in undertaking a reaction. So we have:
- concerted mechanisms, when all bonds in any particular stage of a mechanism are changing in concert via a unique transition state,
- asynchronous concerted mechanism, when all the bonds are changing, but not necessarily all at the same rate and which may involve so called “hidden intermediates”, but which nevertheless stil involves only one transition state.
- stepwise mechanisms, in which more than one transition state is involved, connected by a discrete intermediate along the pathway.
Here I consider an example of another type of (isomerisation) mechanism, involving bond rotations rather than bond formations or breakages. The two bonds in this case have a higher bond order than 1, and so are starting to verge on a type of isomerism known as atropisomerism, where the rotation takes place on a relatively slow time scale (unlike single bonds themselves, where rotation about them is normally relatively fast). Do two such bonds rotate in a stepwise or a concerted manner? In the structure below, we have two rotatable bonds, shown in red and blue, which due to conjugation of the lone electron pair on the nitrogen atoms with the carbonyl group have bond orders >1. Do these bonds rotate in concert or in a stepwise manner?
The calculations of the rotations are done at the B3LYP+GD3+BJ/Def2-SVPP/SCRF=DCM level, Data DOI: 10.14469/hpc/12299
- Firstly, for the system R=R’ = Me. The reaction coordinate is specified around the red bond.
The animation along the IRC (Intrinsic reaction coordinate) appears below, where you can see the red bond rotating and the blue bond spectating.
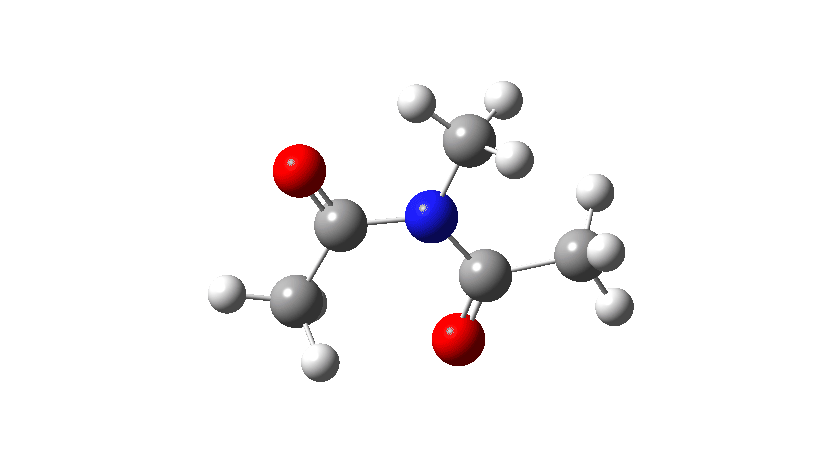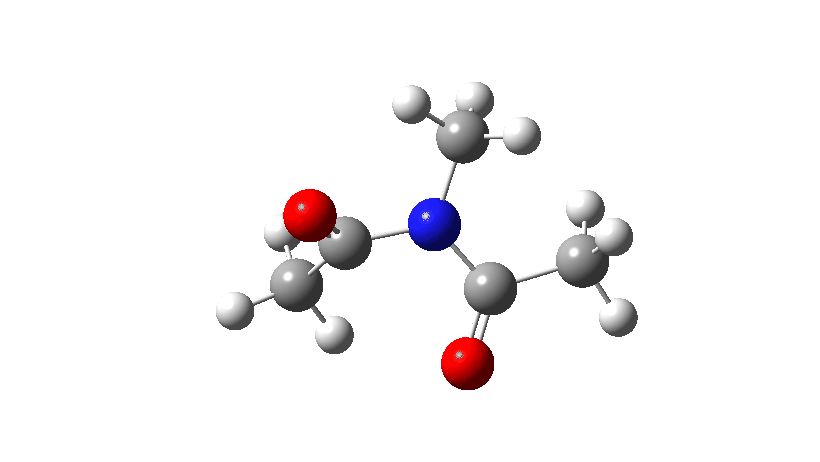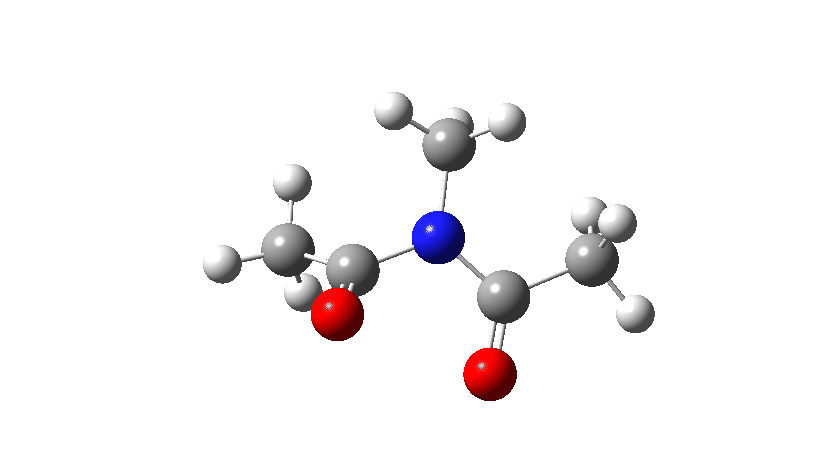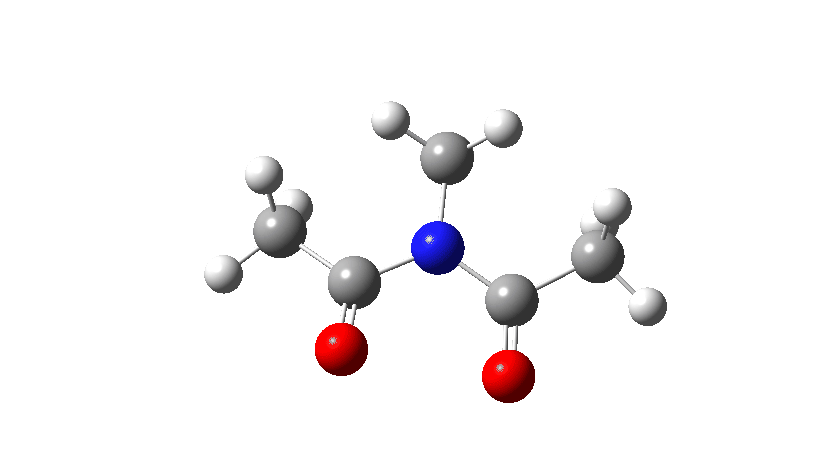
- The response of the dihedral angles about both bonds is shown below, which reinforces the conclusion that whilst one dihedral changes by about 180°, the other hardly changes. The overall dipole moment changes significantly as a result of the relative orientation of the two carbonyl groups changing. The two bonds can be said to rotate in a stepwise mechanism, involving an intermediate where one has rotated and the other has not.
- When the bulk of the central group is increased, different behaviour is now observed.
- Both dihedral angles now change by ~180°, in concert but not in synchrony! The first more or less transforms evenly by ~180°, but the second changes direction at ~IRC=-5 to rejoin the other.
When the steric bulk means that the rotating substituents start to interfere with each other, so-called “gearing” starts to take place where the motions of the two become coupled by the gears. The rotations are now a concerted asynchronous process.
So now to my concluding thought. The above is a simple example of gearing involving rotation about two coupled bonds. So how many bonds can be simultaneously geared so that when one rotates, the others do as well? I am now hunting for an example of three such bonds geared together. And is there a limit to how many can do so in concert? Here we enter into analogy with bond cleavage, where there are numerous examples of bonds breaking in concert, if not in synchrony. Most pericyclic processes are of this type. Is there a similar patten in bond rotations?
Tags: Interesting chemistry
Cyclooctatetraene has a planar transition state, according to Wikipedia (grain of salt needed). Wouldn’t this qualify as a four bond concerted rotation?
Carl, The planar form of cyclo-octatetraene (COT) is indeed planar, as the IRC and animation below show. Looking at the dihedrals around the ring, four do not change (the double bonds) and four do. But the changing dihedrals are not associated with common atoms, unlike the example in the blog.
Thank you for your long and careful answer!
How about cyclohexane chair flip? That one doesn’t proceed through a planar intermediate (I think) and all six bonds rotate synchronously, three in one direction and three in the opposite (again, I think).
The ring flip of cyclohexane is both fascinating and non-trivial. The potential energy surface is shown below
and the key transition state has C2 symmetry and is discussed in my course on conformational analysis at https://www.ch.ic.ac.uk/local/organic/conf/c1_rings.html#a10
The first part of this energy profile is shown below:

The angles along the four unique dihedrals (the remaining two are related by symmetry) are shown below for the numbering:
The first two diagrams show these change more or less linearly by ~ 100-120° along the bonds 6-1 and 1-2 (and 5-6 by symmetry, the C2 axis passes through 6-1).




The next two bonds 2-3 and 3-4 (and 4-5 by symmetry) change non-linearly by only 15-30°
So we can conclude that stating that all six bonds rotate synchronously is not entirely accurate. Three bonds change the dihedral substantially and three much less so. These diagrams above are not to be found in any literature, unless I am mistaken, but it certainly adds a new twist (sorry) to the cyclohexane story!
The source of these data can be found at DOI: https://doi.org/10.6084/m9.figshare.96400.v1
Thank you for the richly substantiated response. I agree that this is fascinating and non-trivial. I hope I will get back to you when I had time to properly think about the twisting motion that may be a simpler (more symmetric?) way to describe the chair flip.