Sometimes you come across a reaction which is so simple in concept that you wonder why it took so long to be accomplished in practice. In this case, replacing toxic ozone O3 as used to fragment an alkene into two carbonyl compounds (“ozonolysis”) by a relatively non-toxic simple nitro-group based reagent, ArNO2 in which the central atom of ozone is substituted by an N-aryl group. As reported by Derek Lowe, two groups have published[1], [2] details of such a reaction (Ar = 4-cyano or 3-CF3,5-NO2). But there are (at least) two tricks; the first is to use photo-excitation using purple LEDs (390nm light) to activate the nitro group. The second is to establish the best aryl substituents to use for achieving maximum yields of the carbonyl compounds and the best conditions for achieving the cyclo-reversion reaction, shown below as TS1. That step requires heating the cyclo-adduct up to ~80° in (aqueous) acetonitrile for anywhere between 1-48 hours. Here I take a computational look at that last step, the premise being that if such a model is available for this mechanism, it could in principle be used to optimise the conditions for the process.
The proposed mechanism for the workup in aqueous acetonitrile[2] is shown below, involving TS1 (a thermal pericyclic cycloreversion reaction), TS2 and TS3 involving intervention of either two or three water molecules to produce the carbonyl compounds and an aryl hydroxylamine (which might of itself be a valuable product). It was also mooted[2] that an alternative mechanism might involve extrusion of an aryl nitrene instead of a cycloreversion (shown as TS4). The calculations use the following method: (U)ωB97XD/Def2-TZVPP/SCRF=acetonitrile. The FAIR data DOI for them is 10.14469/hpc/11269.
Since the workup occurs at up to ~80° in aqueous acetonitrile,[2] the activation free energy that would allow this must be <~25 kcal/mol.
- The first model is a simple closed shell cyclo-reversion, solvated only by the model continuum, giving a barrier (for ethene as substrate) which is a little on the high side for a relatively facile thermal reaction.
- At this level, the nitrene extrusion reaction identifies as a second order saddle-point with a very high energy, eliminating it from possibility for the mechanism.
- Allowing the wavefunction to have some biradical character (TS1 has <S2> before annihilation 0.5534, after 0.0858; a pure biradical for which singlet and triplet states are equal in energy would have a value of 1.00) lowers the energy by a modest 2.5 kcal/mol in this model, but producing a somewhat more realistic free energy barrier.
- Adding 2H2O to the model allows TS2 and TS3 to be directly compared to TS1. The barrier drops a further 3.0 or 4.3 kcal/mol respectively for 2 or 3 waters, and also clearly indicates that TS1 is the rate-limiting step. The barrier corresponds to a reaction which is reasonably fast at ambient or slightly elevated temperatures.
| Model | ΔG‡ TS1 | ΔG‡ TS2 | ΔG‡ TS3 |
|---|---|---|---|
| Reactants | 0 | ||
| Closed shell ionic | 30.0 | – | |
| “TS4” | 73.9 | – | |
| +biradical | 27.5 | – | |
| +biradical + 2H2O | 24.5 | 13.7 | 9.2 |
| +biradical + 3H2O | 23.2 | 12.6 | -1.5 |
| Products + 3H2O | -20.4 | ||
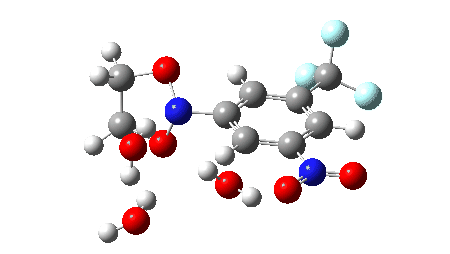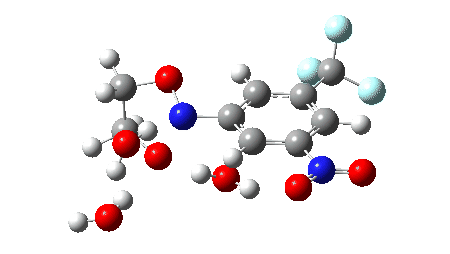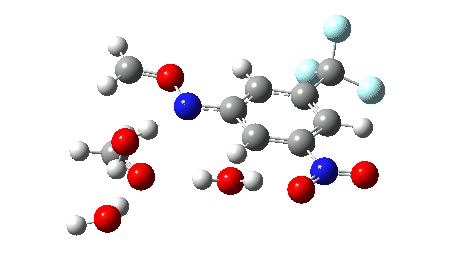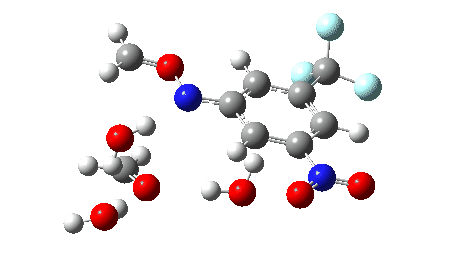
The results here could be used for e.g. computational exploration of how variation in the aromatic group might affect the barrier for cycloreversion.‡ Ideally, a version of this reaction which might operate at much lower temperatures would enhance this alternative to using ozone.
‡ The ΔG‡value for p-CN.3H2O is lower (22.1 kcal/mol vs 23.3 kcal/mol) suggesting it proceeds rather more quickly than the m-CF3,NO2 version. This post has DOI: 10.14469/hpc/11319
References
- D.E. Wise, E.S. Gogarnoiu, A.D. Duke, J.M. Paolillo, T.L. Vacala, W.A. Hussain, and M. Parasram, "Photoinduced Oxygen Transfer Using Nitroarenes for the Anaerobic Cleavage of Alkenes", Journal of the American Chemical Society, vol. 144, pp. 15437-15442, 2022. https://doi.org/10.1021/jacs.2c05648
- A. Ruffoni, C. Hampton, M. Simonetti, and D. Leonori, "Photoexcited nitroarenes for the oxidative cleavage of alkenes", Nature, vol. 610, pp. 81-86, 2022. https://doi.org/10.1038/s41586-022-05211-0
Tags: Interesting chemistry