In previously asking what the largest angle subtended at four-coordinate carbon might be, I noted that as the angle increases beyond 180°, the carbon becomes inverted, or hemispherical (all four ligands in one hemisphere). So what does a search for this situation reveal in the CSD? The query can be formulated as below, in which the distance from the centroid of the four ligands to the central carbon is specified to be in e.g. the range 0.8 to 1.1Å. For tetrahedral carbon surrounded by four carbon ligands, the value would be close to zero, so any value larger than say 0.8Å is worth inspecting.
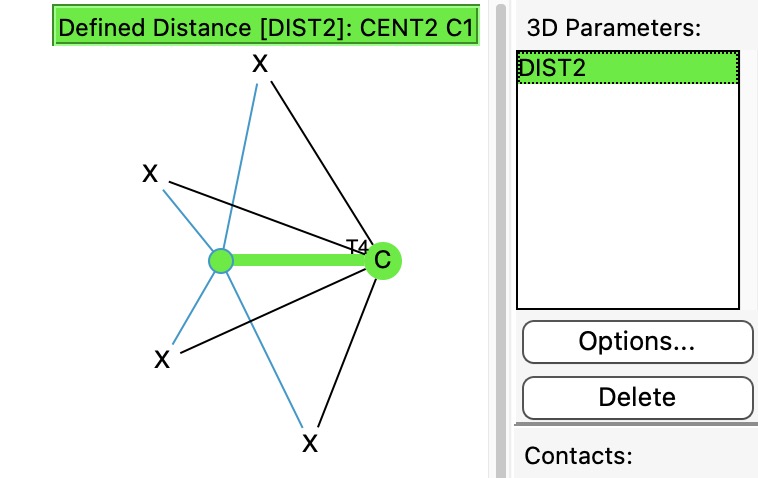
Many of the 101 hits are false positives for inverted carbon (by inspection), but five turn out to be propellanes and eight contain the unusual motif shown below:
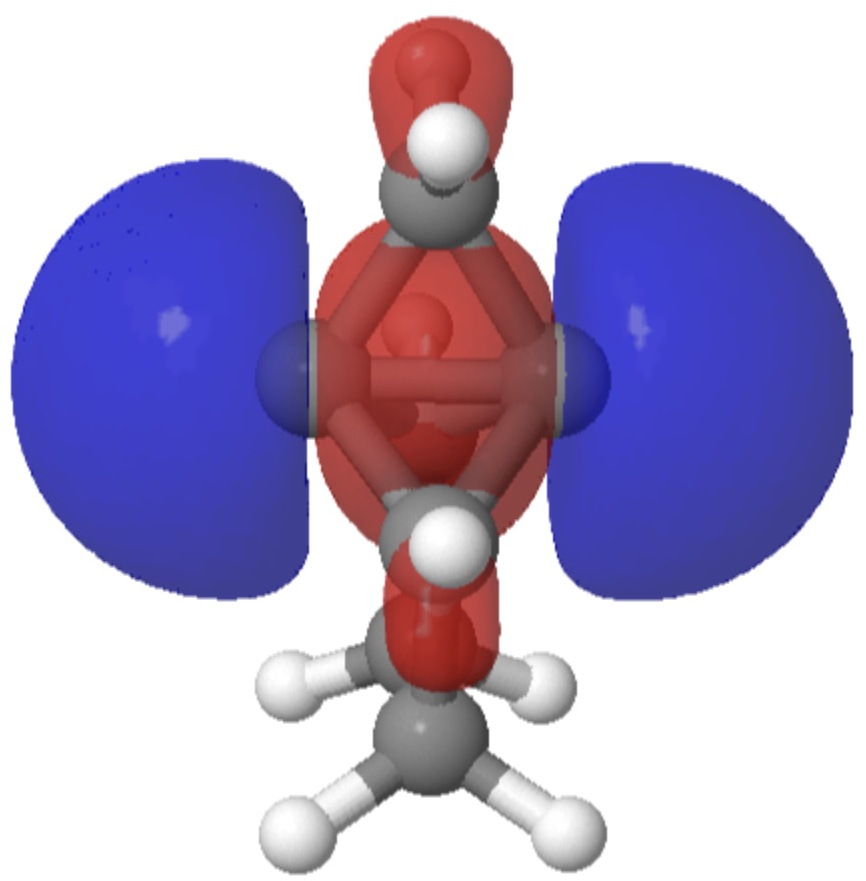
Here I give one example of each. SADHUA[1] is a crystalline [1.1.1]propellane in which the “central” bond length is a normal looking 1.558Å. In fact there is positive (experimental) difference electron density on both “exo” ends of this bond and negative difference density in the “endo” region, suggesting the bond is indeed unusual (FAIR DOI: 10.14469/hpc/11159).
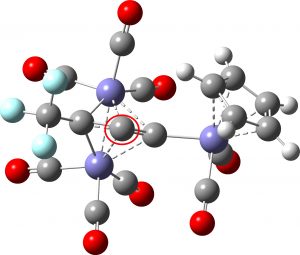
One example of the other motif is SEWZID[2], where the four ligands to the inverted carbon comprise two C-C bonds and two apparent C-Fe bonds of length 2.04Å. A typical C-Fe bond length is in the region 1.8Å, so these are longish C-Fe bonds. Indeed, their Wiberg bond orders emerge as ~0.3, so they would not normally count as a “bond”. Nonetheless, they are indexed as such in the CSD! This highlights an interesting aspect of how to construct a searchable crystal structure database. You have to make a decision on whether any pair of atoms is “bonded” or not. And the decision for bonds with orders <1 can be particularly difficult, especially if calculations of these properties are not part of your assignment toolkit.
So we might conclude that inverted or hemispherical four-coordinate carbon is a rare beast; all the more surprising that the best known examples, the [1.1.1]-propellanes are so stable! Apart from the metallocarbons, one of which is illustrated above, are there any others?
References
- P. Seiler, J. Belzner, U. Bunz, and G. Szeimies, "Crystal Structure and Electron‐Density Distribution of Two [1.1.1] Propellane Derivatives at 81 K", Helvetica Chimica Acta, vol. 71, pp. 2100-2110, 1988. http://dx.doi.org/10.1002/hlca.19880710827
- R. Rumin, F. Petillon, L. Manojlovic-Muir, and K.W. Muir, "Reactions of di- and polynuclear complexes. 6. Reaction of [(.eta.5-C5H5)(CO)Fe{.mu.-C(CF3)=C(CF3)SMe}2Fe(CO)(.eta.5-C5H5)] with [Fe3(CO)12]. Ligand exchange between metals: synthesis and characterization of di- and trinuclear iron-alkyne complexes. Crystal structure of [{Fe(CO)3}2{.mu.-(CF3)CCC[Fe(.eta.5-C5H5)(CO)2]}]", Organometallics, vol. 9, pp. 944-952, 1990. http://dx.doi.org/10.1021/om00118a008
OT, but I once saw a molecular mechanics program minimize a structure into a square pyramid with one carbon at the apex connected to four around the base. This was likely because of a bizarre starting structure, but I don’t recall how the starting structure arose. Perhaps from a bond assignment incorrectly inferred from an old-style PDB file.
The relatively simple nature of the force field functions in most mechanics programs means that such geometries can be allowed as minimisation solutions. They are however considered artefacts of the force field. And yes, the start point could well also be an incorrect bond assignment.