In the previous post, I looked at the intramolecular rearrangement of the oxetane carboxylic acid to a lactone, finding the barrier to the Sn2 reaction with retention was unfeasibly high. Here I explore alternatives.
- This first attempt uses a second molecule of a carboxylic acid (modelled as formic acid for simplicity) to see if it can catalyse the reaction. All FAIR data at 10.14469/hpc/10820
The reaction still occurs with retention at the Sn2 centre, and the free energy barrier is 47.9 kcal/mol (ωB97XD/Def2-TZVPP), very little different from the unimolecular reaction without additional acid (49.9 kcal/mol).
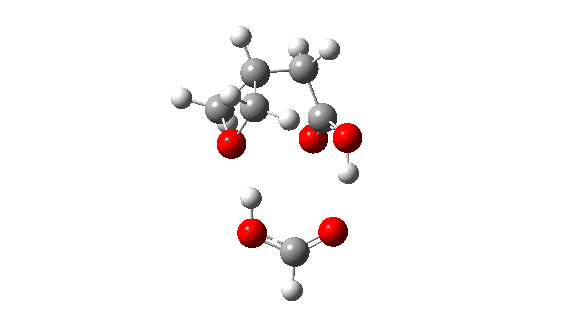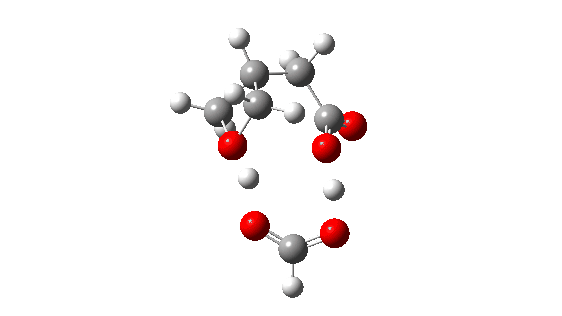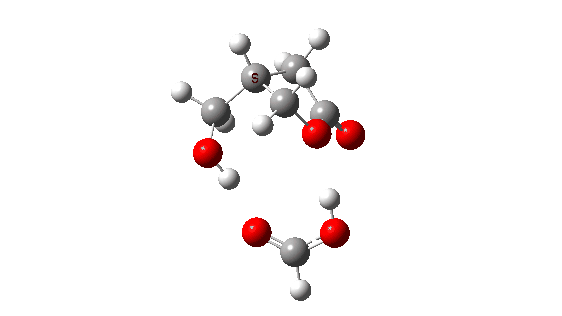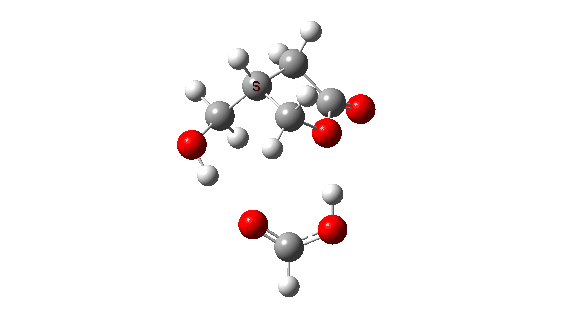
- How about inversion at the Sn2 centre? The energy profile now looks quite wrong, because the additional acid is too small to simultaneously straddle the entire molecule from the point of proton removal to the point of reprotonation, if inversion occurs at the Sn2 centre. The reaction ends up (or starts, depending on your point of view) with a proton in the wrong place!
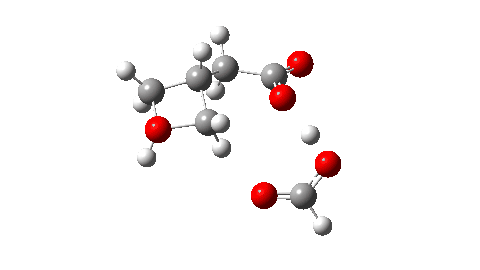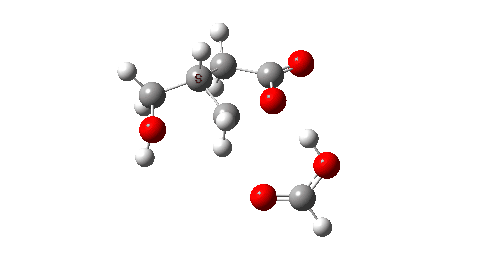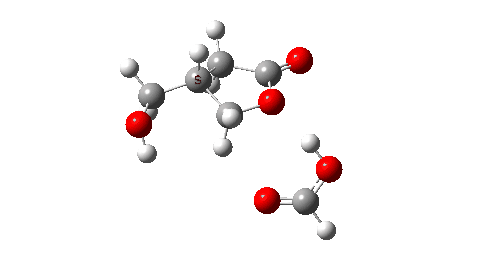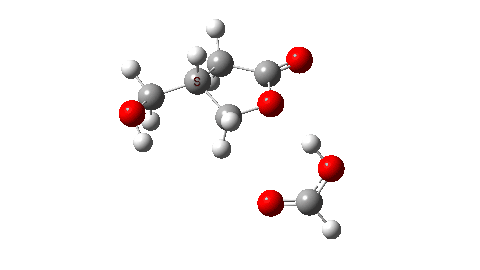
- So the need to make the proton transfer agent larger, by now including TWO additional carboxylic acids.
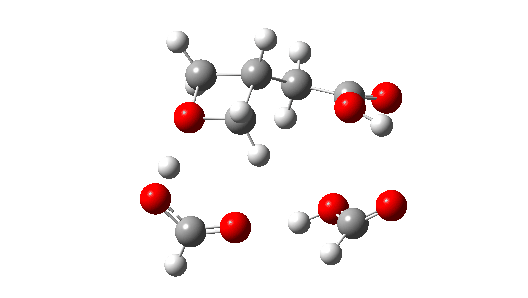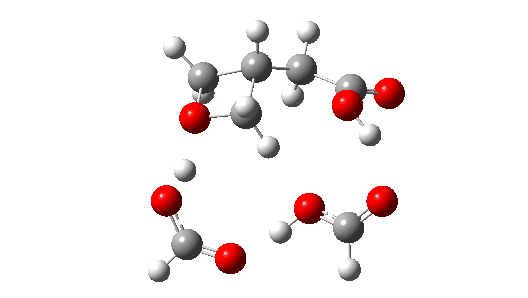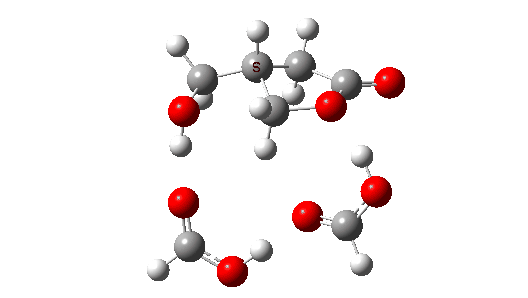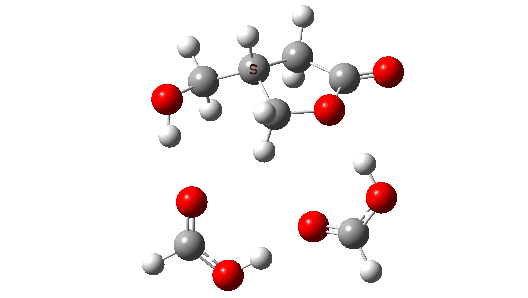
There are now three proton transfers, one at each end of the oxetane-carboxylate and the product lactone and one between the two transfer acids. Watch the animation carefully to note the sequence in which they occur. The free energy barrier is now 27.0 kcal/mol for a standard state of 1 atm (ωB97XD/Def2-TZVPP). This could be reduced (3.2 kcal/mol or more) for the higher effective molar concentrations in the liquid or solid state of the pure oxetane. - The structure of this transition state (click image below to view 3D model) shows one interesting point of CH…O interaction between transfer catalyst and substrate.
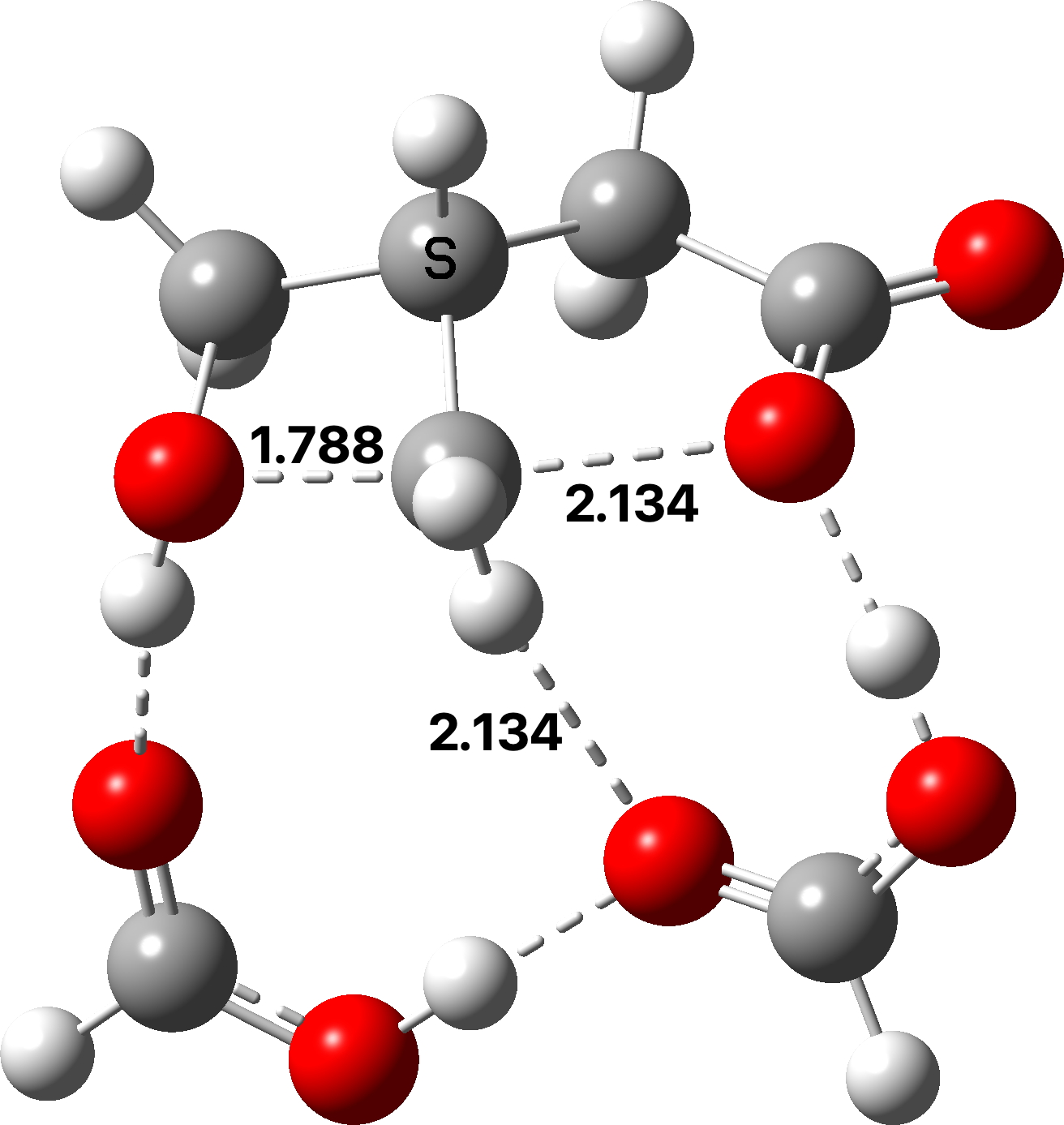
The next steps will be to explore the impact of making substitutions in the oxetane ring; there now seems to be a viable model to use for this purpose.
DOI 10.14469/hpc/10848 and 10.14469/hpc/10862