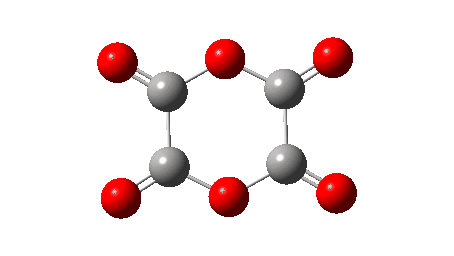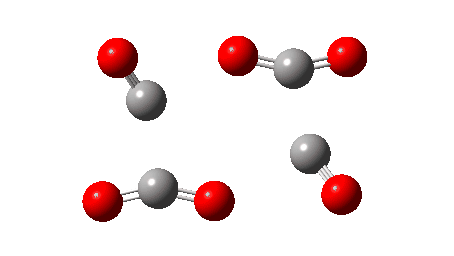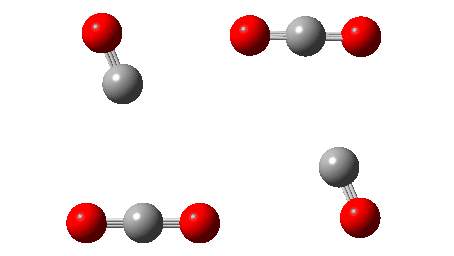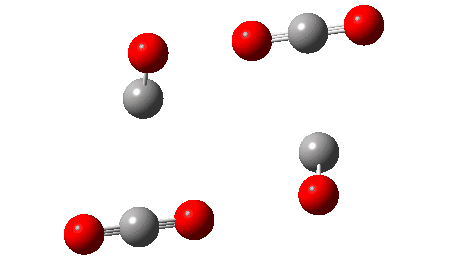
I have long been fascinated by polymers of either carbon dioxide,† or carbon monoxide, or combinations of both. One such molecule, referred to as dioxane tetraketone when it was featured on the ACS molecule-of-the-week site and also known as the anhydride of oxalic acid, or more formally 1,4-dioxane-2,3,5,6-tetraone, has been speculated upon for more than a century.[1]
The history of chemistry has many molecules whose existence has been speculated upon, but where attempted syntheses have failed and for which sound theoretical reasons often only emerged many years later.[2]
The synthesis of dioxane tetraketone was finally achieved in 1998[3] at low temperatures (243K), although it was noted that in CDCl3/Et2O solutions at 273K it quickly decomposed to give equal quantities of carbon monoxide and dioxide. The characterisation was by 13C NMR, for which a single signal at 144.9 ppm was observed. The predicted value using the ACD/CNMR Predictor 2.0 Program (a so-called additive rule-based method) was 154 ppm (the value obtained using a similar tool available in Chemdraw is 150.9 ppm). The monomer oxirane-2,3-dione was also eliminated because of its predicted 13C shift using the same method of 167 ppm (155.3 using Chemdraw). Here I thought I would check these chemical shifts using a DFT-based method and also look at the barrier to the decomposition to see if it corresponds to a facile reaction at 273K (FAIR Data DOI: 10.14469/hpc/10619).
Firstly the NMR, using eg ωB97XD/aug-cc-pvdz/SCRF=chloroform. The calculated value of 148.2 ppm compares well with the observed value of 144.9 ppm. The value calculated for oxirane-2,3-dione was 156.6 ppm, rather lower than the ACD/Predictor method but in agreement with the Chemdraw implementation. The predicted IR spectrum (not reported) is shown below, should it ever be measured for this species.
Next, the reaction energy profile, this time calculated using ωB97XD/Def2-TZVPP for the reaction mechanism shown below.
The IRC reveals that the mechanism (black arrows) is followed, in a concerted process that reveals absolutely no sign of any ionic intermediate (red) which could then lead to oxirane-2,3-dione (blue). The barrier ΔG‡ is 36.9 kcal/mol (it is lower than the total energy inferred below because the entropy is very positive, one molecule being converted to four during the reaction) which is far to high to correspond to a reaction that easily occurs at 273K. The value in water as solvent is very similar, again indicating that the ionic route is not enhanced by a polar solvent. The transition state has another feature of interest. It has C2 chiral symmetry, typical of a pericyclic reaction with Möbius topology, as indeed would be appropriate for an eight electron process.
So what about that mystery then? Well, experimentally dioxane tetraketone decomposes at 273K, which would correspond to a free energy barrier of around 14-15 kcal/mol. The calculated value is far higher, too high to be simply an error in the DFT method. So here is a suggestion. CDCl3, unless very carefully purified, contains HCl, which could very easily catalyse the reaction. So if another solvent were to be tried, lets say acetonitrile in which any trace of acid has been removed, would solutions of dioxane tetraketone then persist at room temperatures for far longer? An experiment perhaps to be tried!
†Perhaps the most fascinating is the cyclic trimer of carbon dioxide, which arguably has pretensions to be aromatic. It has very recently been synthesized.[4],[5]
References
- H. Staudinger, "Oxalylchlorid", Berichte der deutschen chemischen Gesellschaft, vol. 41, pp. 3558-3566, 1908. https://doi.org/10.1002/cber.19080410335
- H.M. Perks, and J.F. Liebman, "Paradigms and Paradoxes: Aspects of the Energetics of Carboxylic Acids and Their Anhydrides", Structural Chemistry, vol. 11, pp. 265-269, 2000. https://doi.org/10.1023/a:1009270411806
- P. Strazzolini, A. Gambi, A.G. Giumanini, and H. Vancik, "The reaction between ethanedioyl (oxalyl) dihalides and Ag2C2O4: a route to Staudinger’s elusive ethanedioic (oxalic) acid anhydride", Journal of the Chemical Society, Perkin Transactions 1, pp. 2553-2558, 1998. https://doi.org/10.1039/a803430c
- M.J. Rodig, A.W. Snow, P. Scholl, and S. Rea, "Synthesis and Low Temperature Spectroscopic Observation of 1,3,5-Trioxane-2,4,6-Trione: The Cyclic Trimer of Carbon Dioxide", The Journal of Organic Chemistry, vol. 81, pp. 5354-5361, 2016. https://doi.org/10.1021/acs.joc.6b00647
- H. Takeuchi, "Geometry Optimization of Carbon Dioxide Clusters (CO<sub>2</sub>)<sub><i>n</i></sub> for 4 ≤ <i>n</i> ≤ 40", The Journal of Physical Chemistry A, vol. 112, pp. 7492-7497, 2008. https://doi.org/10.1021/jp802872p
Tags: Interesting chemistry
I’ve been exploring the idea of decomposing Dioxane tetraketone as a low-gravity propulsion mass, and I am interested in your thoughts on a few facets of the matter. I’m admittedly not even close to where I should be in regards to my chemistry, but I can’t help but wonder. Since I’m uneducated, I will ask that you pardon my avoiding erroneous jargon by simplifying my thoughts.
First is trapping atmospheric CO2 in the form of C4O6. In my little brain, that’s cool down some air, isolate the CO2, and then you should be able to take a ratio of (4) CO2 to (2) H2, and convince 2 of the Oxygens to jump ship into water vapor. If you’re at low enough temperatures, you should be able to use the energy from that reaction to briefly heat the (2) CO2 and (2) CO, and in a persistent reaction that evacuates the water ice by virtue of tiny thermal convection motions you should end up with a bunch of water ice on top of a pile of C4O6 with a thin barrier of liquid CO2 that’s reacting.
What am I getting wrong?
Part two of this “not even wrong” string of crackpottery assumes that we’ve managed to hold our C4O6 at stable cryogenic temperatures. What part of chemistry do I need to know in order to design my nozzles and valves for a specific impulse? How fast does C4O6 decompose, into what densities within, say, a cubic liter? I simply don’t know what decides these things, so I’m taking a moment to ask a random blog I found — because google is no help at all.
I hope this finds you well!