Minds (and memories) can work in wonderful ways. In 1987[1] we were looking at the properties of “stable” tetrahedral intermediates formed in carbonyl group reactions. The reaction involved adding phenylhydroxylamine to acetyl cyanide. NMR signals for two new species were detected, and we surmised one was due to N-attack on the carbonyl and one was due to O-attack, in each case to form a stable tetrahedral intermediate. To try to identify which was which, 15N labelled hydroxylamine was used and then the 15N-13C coupling constants were measured, which could either be 1-bondJ (for N-attack) or 2-bondJ (for O-attack).
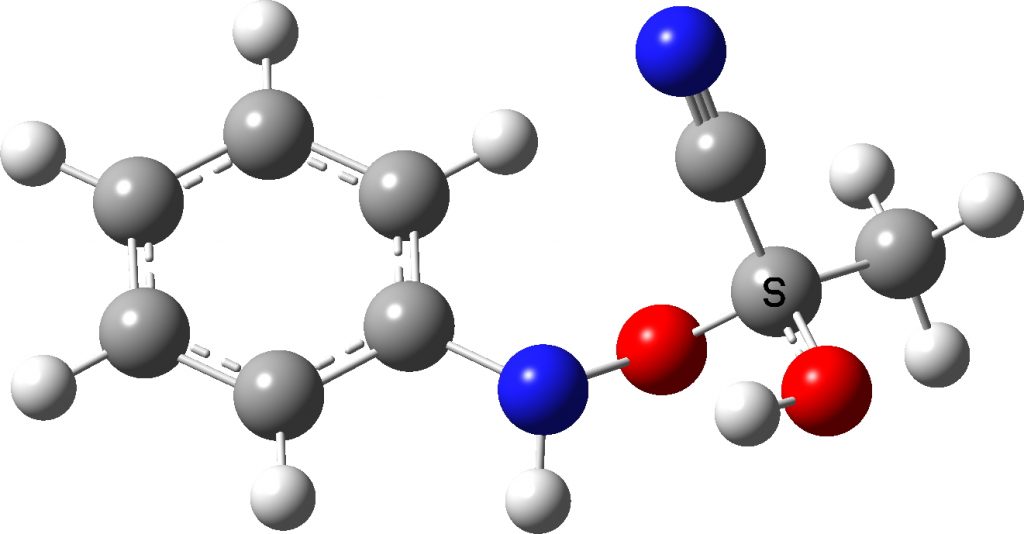
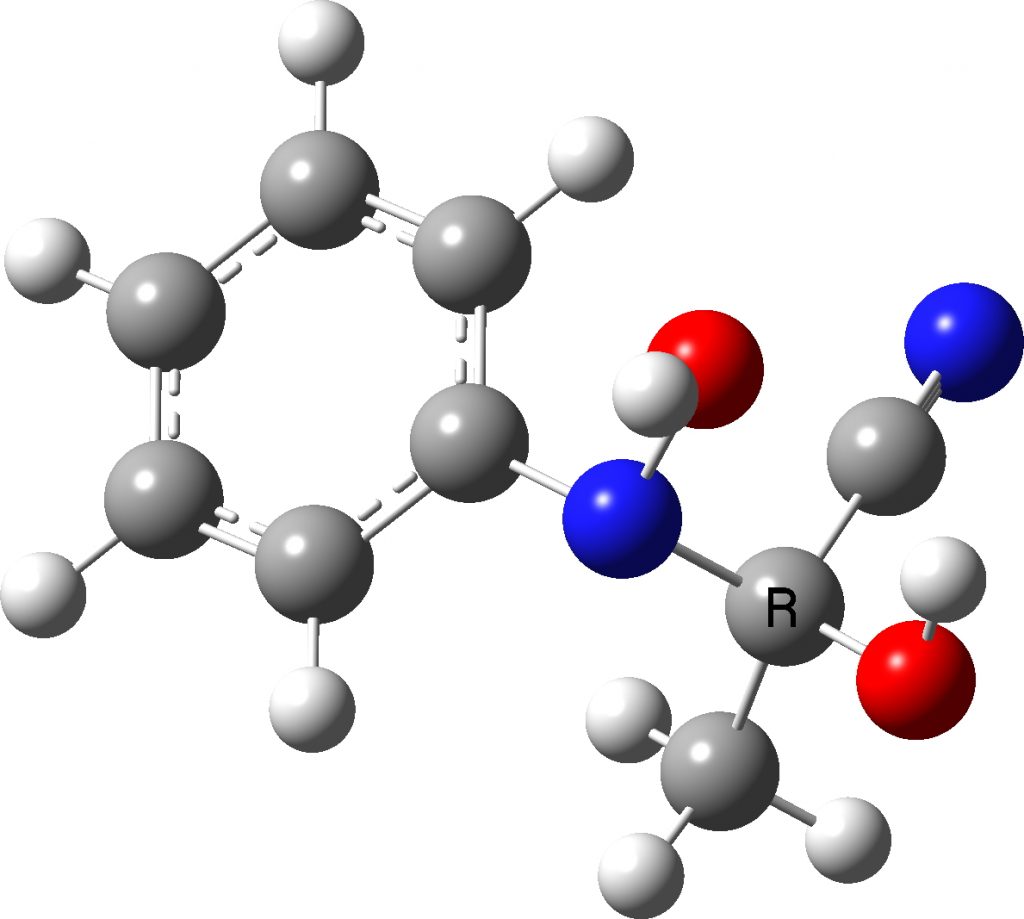
Well, 35 years later, literally in a dream on the morning of 7th June, 2022, these results came back to me and the dream involved wondering whether we had gotten the assignments of the N- and O-species the correct way around. You see we had assigned the larger of the 15N-13C couplings to the two bond (O-attack, species 3 below) rather than one-bond (N-attack, species 4 below) coupling. In 1987, the art of accurately computing such couplings was still in its infancy, but now in 2022 it is quick and easy to do. So here I report the results, which 35 years on allows a check of those assignments.
The necessary calculations are assembled at FAIR DOI: 10.14469/hpc/10593 conducted at the ωB97XD/aug-cc-pvdz/scrf=acetonitrile level. Firstly, it is important that the conformational space of these molecules is explored, since they contain a plethora of interesting anomeric effects. I will not discuss this process, simply quoting what I believe to be the lowest energy conformation for both isomers.
| # | Property | Species 3 | Species 4 |
|---|---|---|---|
| 1 | ΔG298 | -608.600542 | -608.598472 |
| 2 | ΔG215 | -608.586956 | -608.585163 |
| 3 | NBO E(2) | 14.3,19.4,10.9,8.1 | 10.0,11.2,9.9 |
| 4 | δC obs | 94.3 ppm | 85.0 ppm |
| 5 | δC calc | 97.2 (Δδ 2.9) | 88.1 (Δδ 3.1) |
| 6 | JN-C obs | 2J ±2.5 Hz | 1J ±1.3 Hz |
| 7 | JN-C calc | 2J +1.7 | 1J +0.8 |
- The relative free energies ΔΔG298 favour 3 over 4 by 1.3 kcal/mol at 298K (9:1). The article notes that 3 is significantly favoured over 4 at higher temperatures (i.e. ~298K) but that the concentration of 4 increases at lower temperatures.
- At 215K, ΔΔG215 reduces to 1.1 kcal/mol, but this equates to 13:1 at this temperature. ΔΔG215 would need to be about 0.8 kcal/mol for 4 to increase (6.5:1), but these are small errors in energy and a more accurate calculation would have to be done to get this aspect correct.
- The NBO E(2) terms indicating overlap between a lone pair and an acceptor orbital (the anomeric effect), show a dazzling variety of interactions for such a small molecule. Species 3 shows four significant interactions, species 4 one less.
- The chemical shifts measured for 3 and 4 –
- – are matched by the calculation, the error being similar for both species.
- The 15N-13C coupling constants –
- – are again matched, with the 1J coupling being about half the value of the 2J coupling for both obs and calculated values.
The nature of modern scientific research, and the funding available for it, means that old work is rarely re-investigated using more recent techniques. In this case, the reinvestigation does not require the molecules to be re-synthesized again, merely that a retrospective computational layer be applied. As a result of my dream of four days ago, this process has produced an interesting new layer which thankfully confirms the original conclusions.
References
- A.M. Lobo, M.M. Marques, S. Prabhakar, and H.S. Rzepa, "Tetrahedral intermediates formed by nitrogen and oxygen attack of aromatic hydroxylamines on acetyl cyanide", The Journal of Organic Chemistry, vol. 52, pp. 2925-2927, 1987. https://doi.org/10.1021/jo00389a050
Tags: Interesting chemistry