Normally, aromaticity is qualitatively assessed using an electron counting rule for cyclic conjugated rings. The best known is the Hückel 4n+2 rule (n=0,1, etc) for inferring diatropic aromatic ring currents in singlet-state π-conjugated cyclic molecules‡ and a counter 4n rule which infers an antiaromatic paratropic ring current for the system. Some complex rings can sustain both types of ring currents in concentric rings or regions within the molecule, i.e. both diatropic and paratropic regions. Open shell (triplet state) molecules have their own rule; this time the molecule has a diatropic ring current if it follows a 4n rule, often called Baird’s rule. But has a molecule which simultaneously follows both Hückel’s AND Baird’s rule ever been suggested? Well, here is one, as indeed I promised in the previous post.
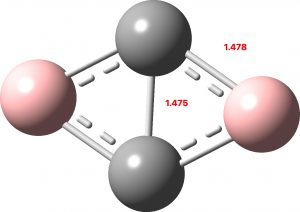
The species shown above has two carbons and two borons in a ring. These have a total of 14 valence electrons, eight of which occupy the C-B bonds, leaving six contributing to circulating ring currents. These partition into two π-electrons which then form a Hückel 4n+2 aromatic (n=0) and four σ-electrons which then form a Baird 4n aromatic (n=1) as a triplet. The triplet for this molecule is indeed its lowest state, 38.9 kcal/mol or 45.4 kcal/mol in free energy lower than the two lowest energy singlet states. These arise by placing two electrons in either of the two orbitals σ2 or σ3 each singly occupied in the triplet state (FAIR Data collection: 10.14469/hpc/10267)
| Bonding MOs for C2B2. Click image to load 3D model |
|
|---|---|
| σ3 | σ2 |
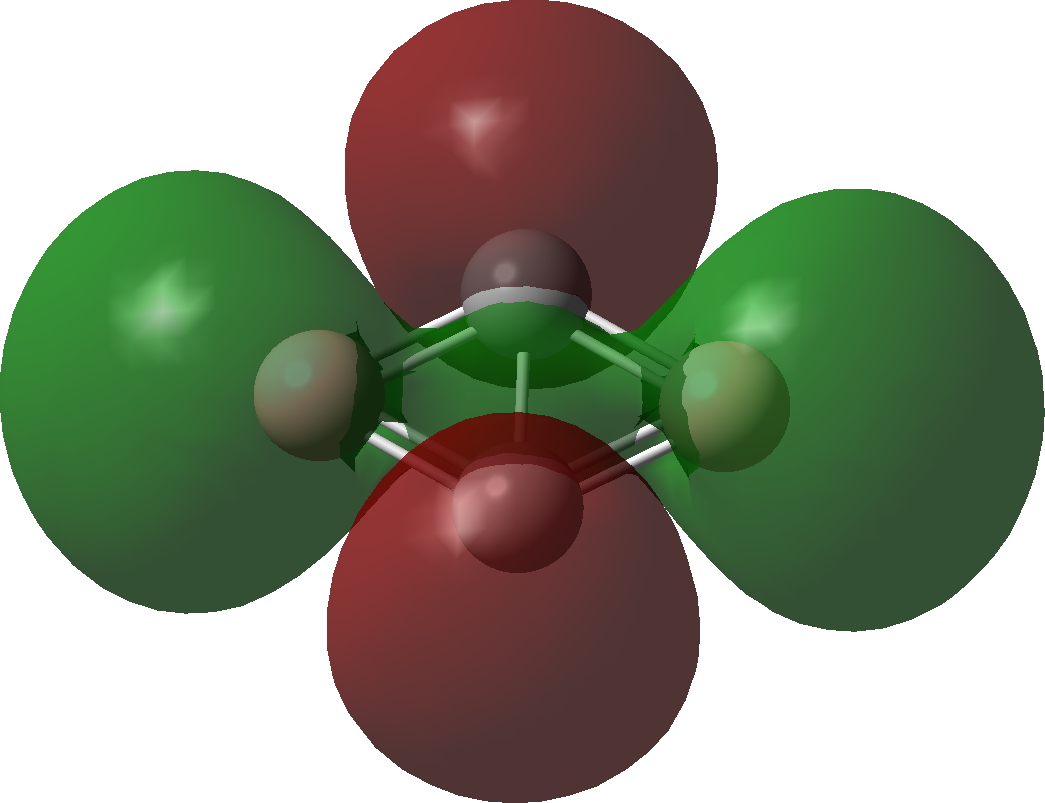 |
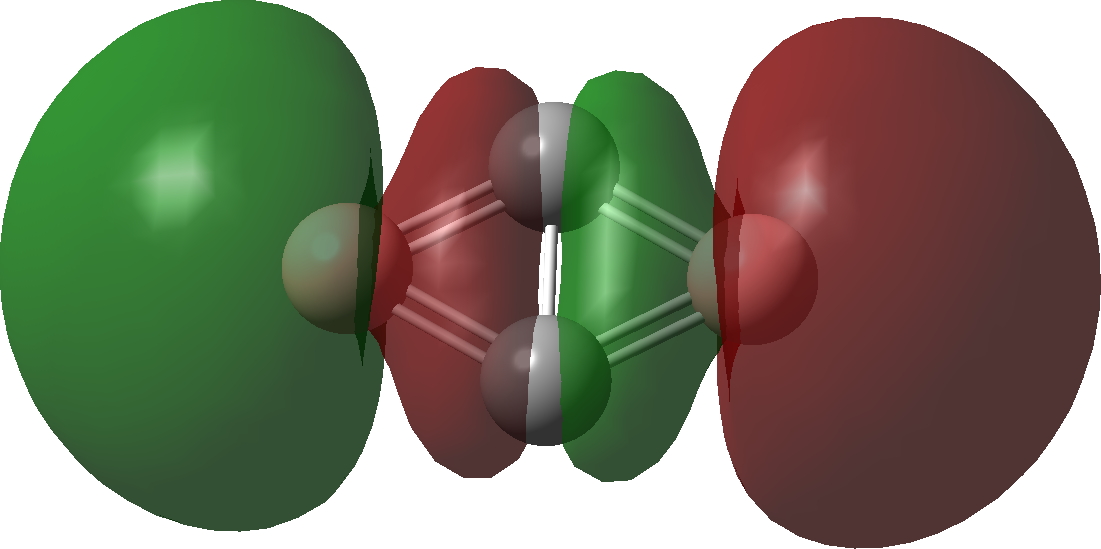 |
| σ1 | |
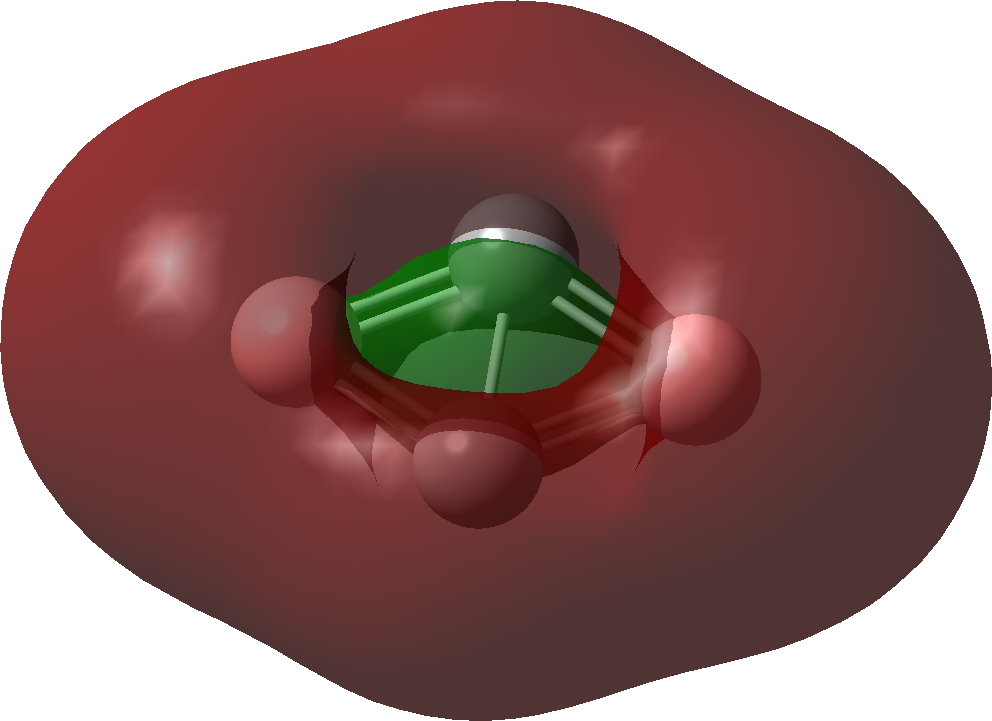 |
|
| π1 | |
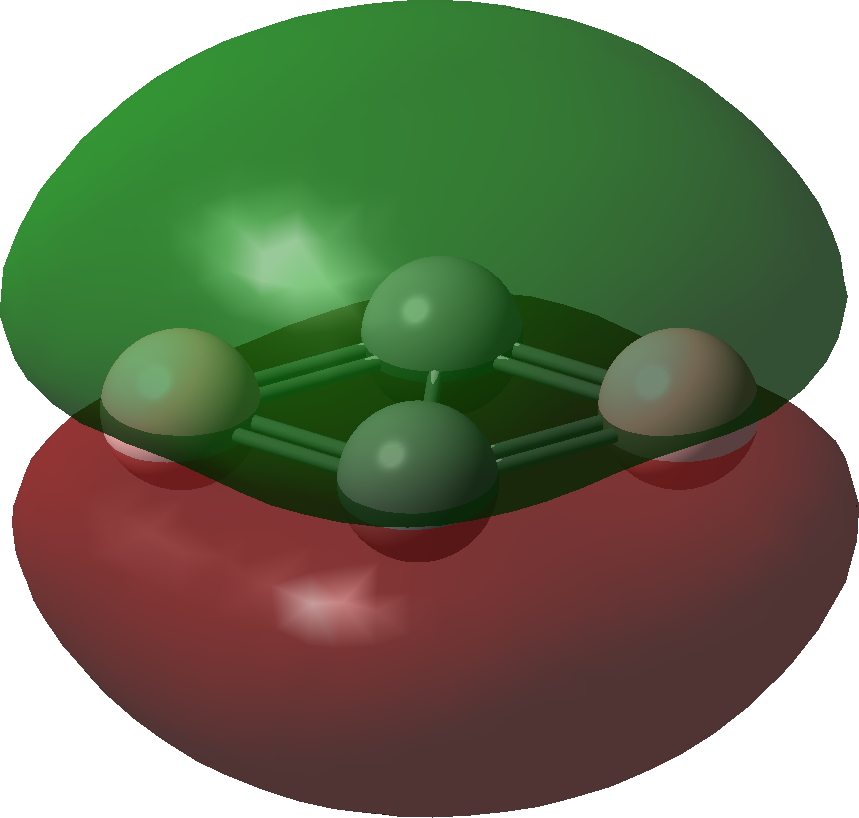 |
|
So here we see a different sort of doubly aromatic molecule, to add to C4, B4 and N4. With two electrons less than C4, it is now doubly aromatic as a triplet state, this time conforming to two different electron counting rules. It would be good to know if any other examples showing this pattern are known.
‡Hückel’s rule originally applied to p-π electrons in a cycle, such as benzene. Nowadays it is also used for σ in-plane electrons in a cycle.
This post has DOI: 10.14469/hpc/10271.
Interesting post Henry! This phenomenon with different aromaticity from different electron counting rules for sigma/pi are known for all-metal clusters at least since this publication by Solà in 2011: https://doi.org/10.1007/s00214-010-0805-8. Then you also have d and f electrons, so possibly you can extend the concept even further. I think your system is a bit unusual in that it would have the triplet localized in the sigma system.
[…] earlier examples of 4 atom programs displaying two layers of aromaticity illustrated how 4 (B4), 8 (C4) and 12 (N4) […]