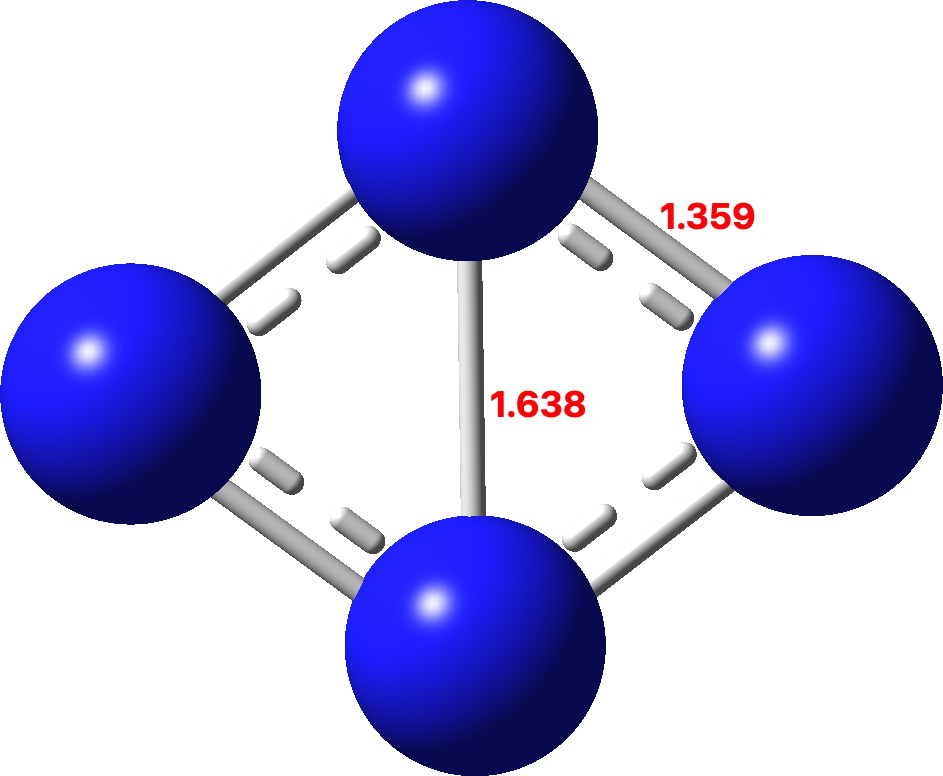
I discussed in the previous post the small molecule C4 and how of the sixteen valence electrons, eight were left over after forming C-C σ-bonds which partitioned into six σ and two π. So now to consider B4. This has four electrons less, and now the partitioning is two σ and two π (CCSD(T)/Def2-TZVPPD calculation, FAIR DOI: 10.14469/hpc/10157). Again both these sets fit the Hückel 4n+2 rule (n=0).
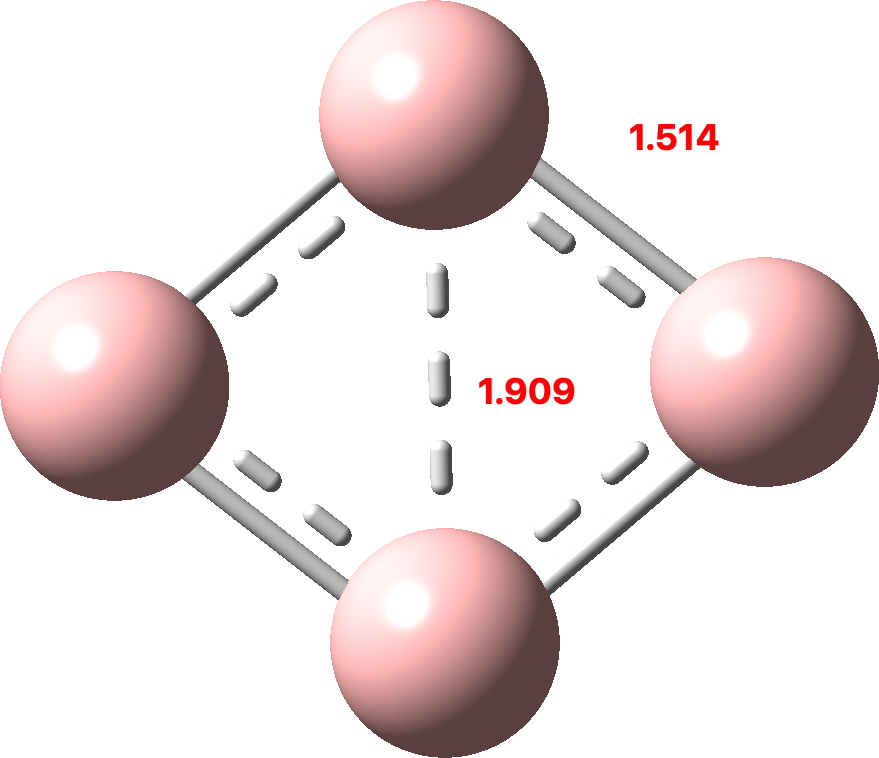
Since B4 has only two rather than six delocalized σ-orbitals, the contributions to the central B-B bond are weaker and so the B-B bond is much longer.
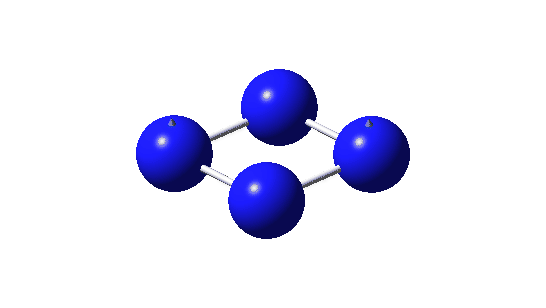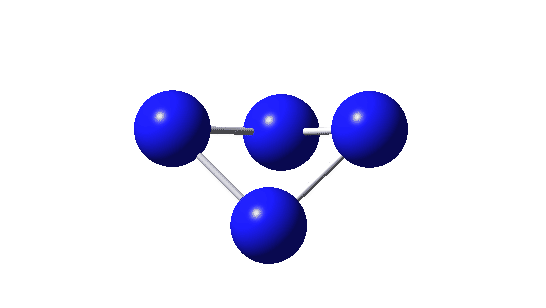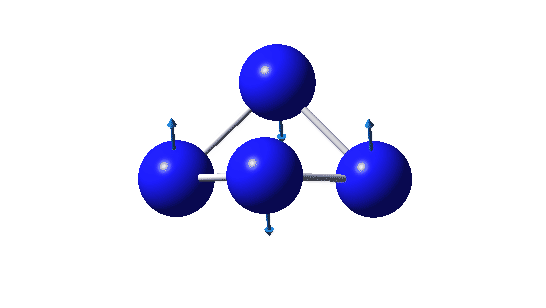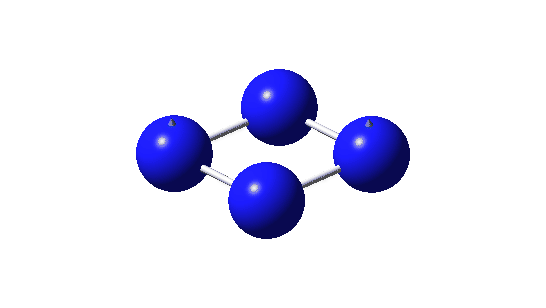
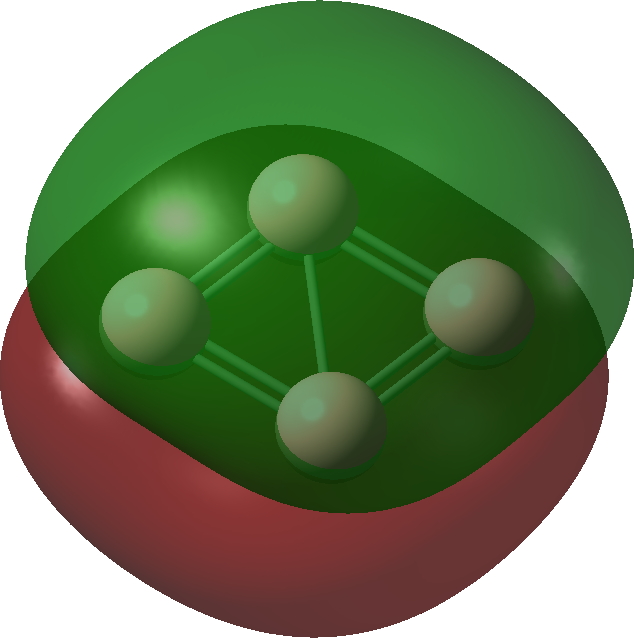
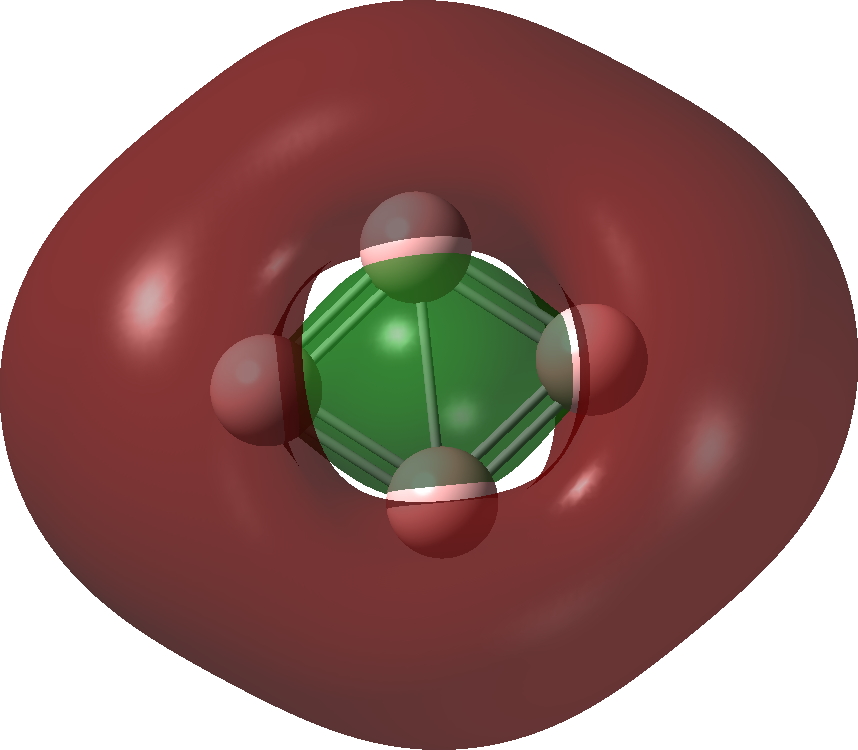
| Bonding MOs for B4. Click image to load 3D model |
|
|---|---|
| σ1, -0.335 au | |
 |
|
| π1, -0.372 au | |
 |
| π-Bonding MOs for N4. Click image to load 3D model |
|
|---|---|
| π3 | π2 |
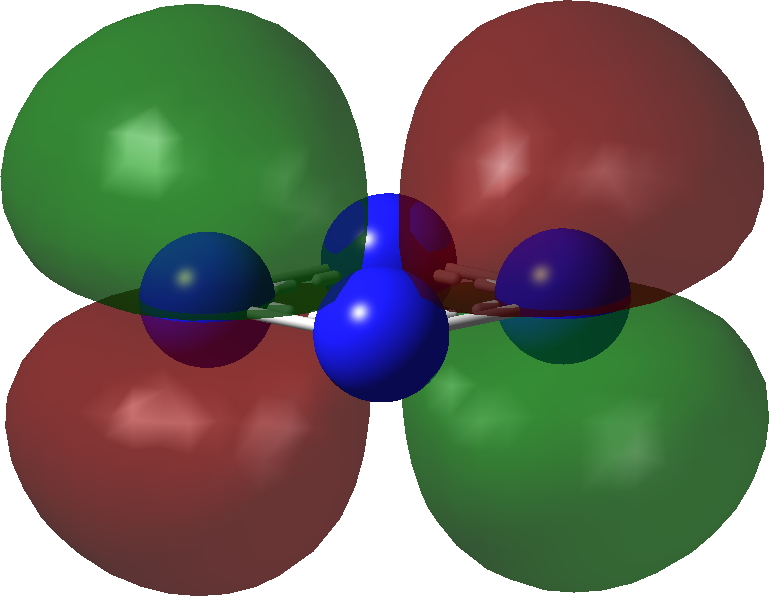 |
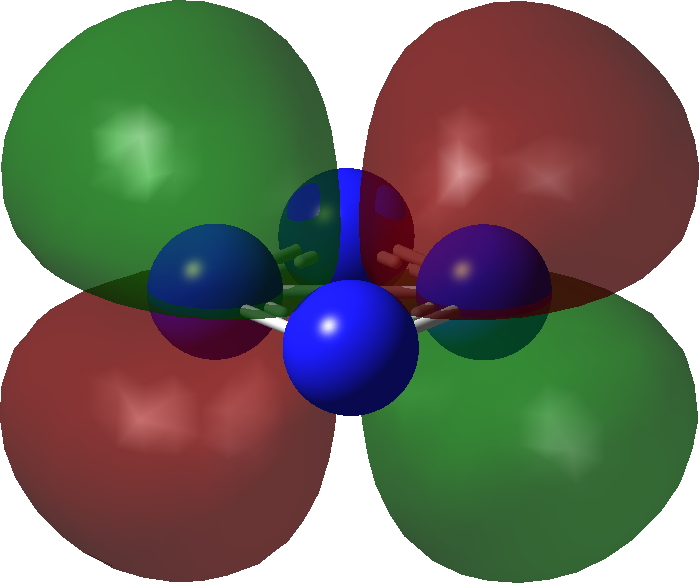 |
| π1 | |
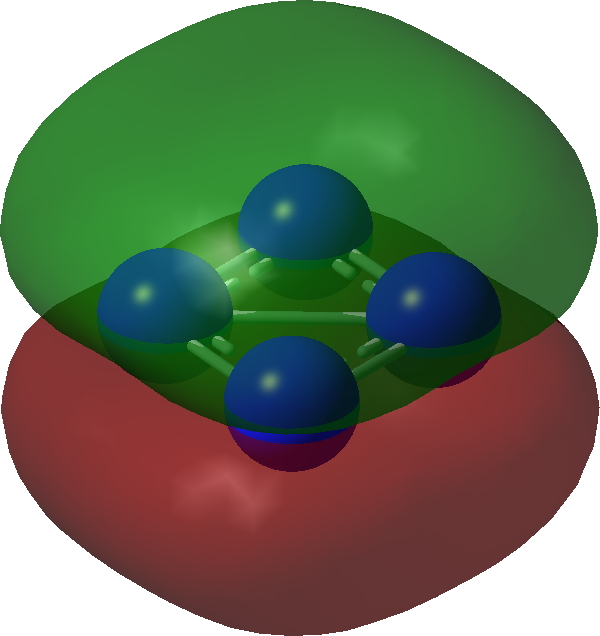 |
|
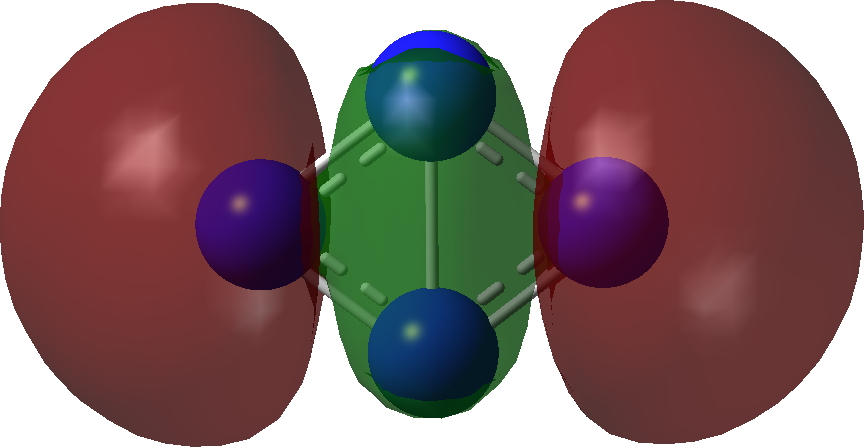
| σ-Bonding MOs for N4. Click image to load 3D model |
|
| σ3 | σ2 |
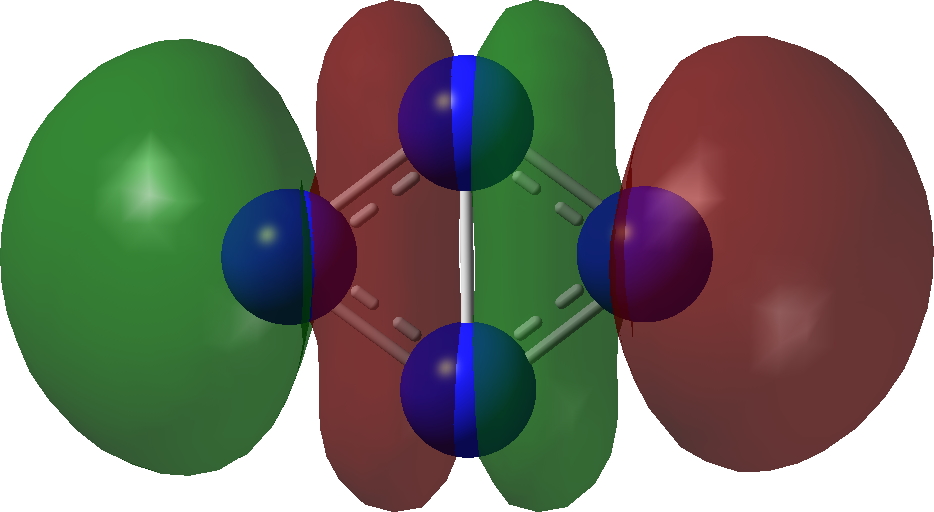 |
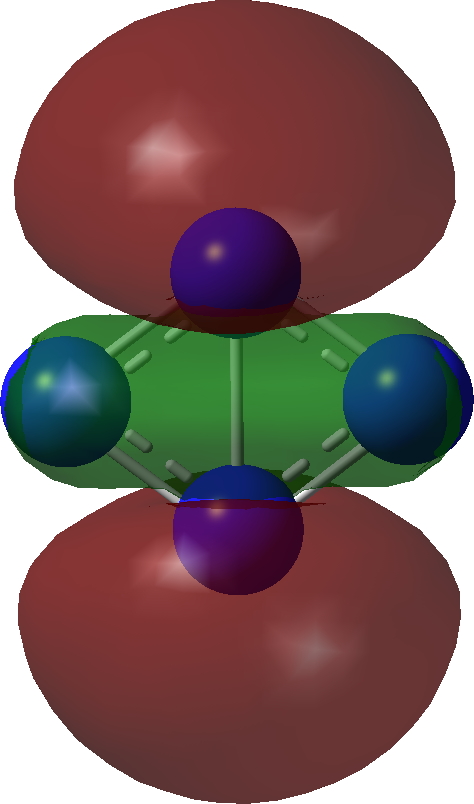 |
| σ1 | |
 |
|
The pattern for N4 is different in several aspects. Firstly the π-system has six bonding electrons distributed over only four atoms. This makes the electron repulsions too high and the species is no longer stable, having one large imaginary force constant corresponding to an out-of-plane distorsion. Secondly the lowest energy σ orbital is highly localised onto two nitrogens rather than being delocalised around the ring periphery. So all those electrons crammed into a small space have taken their toll.
Thus far we have identified three species, B4, C4 and N4 with interesting sets of respectively 4,8 and 12 electrons, all partitioned into 4n+2 collections. But what happens if one cannot do that; lets say 6 and 10 electrons? Hang around to find out!